AIM2 drives inflammatory cell death and monkeypox pathogenesis
- PMID: 41225161
- PMCID: PMC12661009
- DOI: 10.1038/s41423-025-01367-7
AIM2 drives inflammatory cell death and monkeypox pathogenesis
Abstract
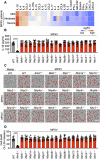
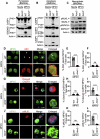
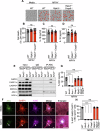
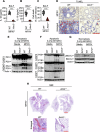
Monkeypox, a zoonotic disease caused by the monkeypox virus (MPXV), has significant global public health implications. Inflammasomes serve as crucial components of the innate immune system, detecting pathogens and triggering cell death in infected cells to eliminate harmful agents. However, the precise molecular mechanisms governing the activation of inflammasomes during MPXV infection remain largely unclear. Using CRISPR-knockout cytosolic innate immune sensor screening, we identified AIM2 as the sensor for MPXV within the inflammasome, a trigger for inflammatory cell death. Mechanistically, AIM2 forms a complex with essential cell death molecules, including ASC and caspase-1 (CASP1), without interacting with RIPK3 or CASP8. Loss of ASC, CASP1, or gasdermin D (GSDMD) reduced cell death following MPXV infection, whereas loss of GSDME, CASP3, CASP6, CASP7, CASP9, RIPK3, or MLKL did not. Pyroptotic cell death was predominantly observed in infected cells, whereas apoptotic and necroptotic signaling pathways were primarily activated in uninfected bystander cells. Furthermore, we found that the transcription factor IRF1 serves as an upstream regulator of AIM2, controlling AIM2-dependent cell death. In experiments involving AIM2-deficient mice infected with MPXV, we observed a decrease in proinflammatory cytokines, multiple inflammatory cell death pathways, and leukocyte migration, culminating in increased viral spread. CAST/EiJ mice succumbed to high-dose MPXV infection within 8 days, whereas AIM2 inhibition increased survival, with 10% of the mice treated with an AIM2 inhibitor surviving the infection. In a low-dose infection model, AIM2 inhibition reduced IL-1β and IL-18 production, LDH release, and tissue pathology. These findings highlight the critical role of AIM2-mediated inflammasome activation, along with multiple programmed cell death pathways, in shaping the innate immune response to MPXV infection, offering valuable insights for developing therapeutic strategies targeting AIM2 and the broader innate immune response against monkeypox.
Keywords: AIM2; Inflammasome; Inflammation; Inflammatory cell death; Innate immunity; Monkeypox virus.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. - PubMed
MeSH terms
Substances
Grants and funding
- 2022R1C1C1007544/National Research Foundation of Korea (NRF)
- 2024M3A9H5043152/National Research Foundation of Korea (NRF)
- RS-2022-KH128422(HV22C015600/Korea Health Industry Development Institute (KHIDI)
- HR22C1363/Korea Health Industry Development Institute (KHIDI)
- 1.220107.01/Ulsan National Institute of Science and Technology (UNIST)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

