Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status
- PMID: 41226410
- PMCID: PMC12607542
- DOI: 10.3390/ijms262110371
Potassium-Hydroxide-Based Extraction of Nicotinamide Adenine Dinucleotides from Biological Samples Offers Accurate Assessment of Intracellular Redox Status
Abstract
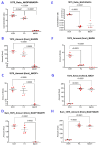
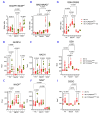
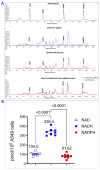
The reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) is a primary electron donor for both antioxidant enzymes, such as glutathione reductase, and pro-oxidant enzymes, such as NADPH oxidases that produce reactive oxygen species (ROS) and nitric oxide synthases that generate nitric oxide which act as signaling molecules. Monitoring NADPH levels, NADPH/NADP+ ratio, and especially distinguishing from NADH, provides vital information about cellular redox status, energy generation, survival, lineage specification, and death pathway selection. NADPH detection is key to understanding metabolic reprogramming in cancer, aging, and cardiovascular, hormonal, neurodegenerative, and autoimmune diseases. Liquid chromatography combined with mass spectrometry (LC-MS) is crucial for NADPH detection in redox signaling because it offers the high sensitivity, specificity, and comprehensive profiling needed to quantify this vital but labile redox cofactor in complex biological samples. Using hepatoma cell lines, liver tissues, and primary hepatocytes from mice lacking transaldolase or nicotinamide nucleotide transhydrogenase, or having lupus, this study demonstrates that accurate measurement of NADPH depends on its preservation in reduced form which can be optimally achieved by extraction of metabolites in alkaline solution, such as 0.1 M potassium hydroxide (KOH) in comparison to 80% methanol (MeOH) alone or 40:40:20 methanol/acetonitrile/formic acid solution. While KOH extraction coupled with hydrophilic interaction liquid chromatography (HILIC) and mass spectrometry most reliably detects NADPH, NADP, NADH, NAD, polyamines, and polyols, MeOH extraction is best suited for detection of glutathione and overall discrimination between complex metabolite extracts. This study therefore supports performing parallel KOH and MeOH extractions to enable comprehensive metabolomic analysis of redox signaling.
Keywords: NAD; NADH; NADP; NADPH; alkaline extraction; formic acid extraction; hepatocellular carcinoma; hepatocyte; liver; lupus; methanol extraction; transaldolase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

