Cellular Metabolic Responses to Copper Nanoparticles: Comparison Between Normal and Breast Cancer Cells
- PMID: 41226752
- PMCID: PMC12609715
- DOI: 10.3390/ijms262110716
Cellular Metabolic Responses to Copper Nanoparticles: Comparison Between Normal and Breast Cancer Cells
Abstract
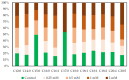
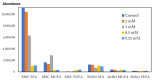
The use of copper nanoparticles (CuNPs) seems to be an alternative therapeutic strategy for cancer therapy due to low-cost synthesis and anticancer activity. In this work, CuNPs' effects were tested in various concentrations on two types of cells: mesenchymal stem cells (MSCs) and a breast cancer cell line, SKBR3. The concentrations (0.25 mM, 0.5 mM, 1 mM and 2 mM) were first tested on an impedance-based cytotoxicity assay and then used in further cellular metabolic assays. Next, several techniques were applied to test the chosen concentrations: assessment of apoptosis, intracellular reactive oxygen species (ROS) levels, oxidative stress-related gene expression, assessment of mitochondrial respiration and fatty acid methyl ester (FAME) profile evaluation. The higher CuNP concentrations tested on the SKBR3 cell line showed a dose-dependent decrease in the cell index. SKBR3 cells displayed increased CAT and SOD expression, revealed by strong dose-dependent fluorescence. Annexin/PI staining confirmed increased SKBR3 cell death induced by the higher doses of CuNPs. SKBR3 revealed higher baseline respiratory capacity compared to MSCs. Fatty acid methyl esters (FAMEs) are in higher abundance in MSCs compared to the SKBR3 cell line. The different metabolic response in the tested cells to the CuNPs' presence could help establish a future personalized treatment for breast cancer patients.
Keywords: in vitro toxicity; metabolomics; nanomaterials; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Reddy K.R. Green Synthesis, Morphological and Optical Studies of CuO Nanoparticles. J. Mol. Struct. 2017;1150:553–557. doi: 10.1016/j.molstruc.2017.09.005. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

