O-GlcNAcylation: A Nutrient-Sensitive Metabolic Rheostat in Antiviral Immunity and Viral Pathogenesis
- PMID: 41227388
- PMCID: PMC12610142
- DOI: 10.3390/cells14211743
O-GlcNAcylation: A Nutrient-Sensitive Metabolic Rheostat in Antiviral Immunity and Viral Pathogenesis
Abstract
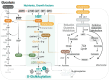
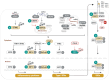
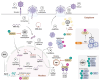
Viruses account for the most abundant biological entities in the biosphere and can be either symbiotic or pathogenic. While pathogenic viruses have developed strategies to evade immunity, the host immune system has evolved overlapping and redundant defenses to sense and fight viral infections. Nutrition and metabolic needs sculpt viral-host interactions and determine the course and outcomes of the infection. In this review, we focus on the hexosamine biosynthesis pathway (HBP), a nutrient-sensing pathway that controls immune responses and host-viral interactions. The HBP converges on O-GlcNAcylation, a dynamic post-translational modification of cellular proteins, that emerged as a critical effector of immune cell development, differentiation, and effector functions. We present a broad overview of uncovered O-GlcNAc substrates identified in the context of viral infections and with a functional impact on antiviral immunity and viral restriction, or conversely on exacerbating viral-induced pathologic inflammation or viral oncogenesis. We discuss the clinical implications of these findings, current limitations, and future perspectives to harness this pathway for therapeutic purposes.
Keywords: glycosylation; host–pathogen interaction; immunometabolism; infection; inflammation; innate immunity; nutrient sensing; pattern-recognition receptors; post-translational modification; virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Roychoudhury S., Das A., Sengupta P., Dutta S., Roychoudhury S., Choudhury A.P., Ahmed A.B.F., Bhattacharjee S., Slama P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health. 2020;17:9411. doi: 10.3390/ijerph17249411. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

