Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers
- PMID: 41228300
- PMCID: PMC12609813
- DOI: 10.3390/cancers17213507
Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers
Abstract
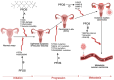
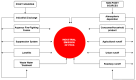
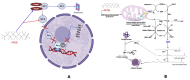
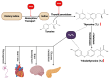
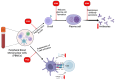
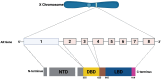
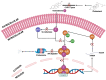
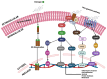
Environmental pollution, driven by industrialization, urbanization, and agricultural practices, has intensified global ecological degradation. Among the most concerning pollutants is PFOS, a synthetic compound known for its chemical stability, environmental persistence, and bioaccumulative potential. Widely utilised in industrial and consumer products, PFOS infiltrates ecosystems and food chains, posing substantial risks to human and animal health. Upon exposure, PFOS disrupts lipid metabolism, damages cellular membranes, and alters signaling pathways through partial metabolism by cytochrome P450 enzymes. Accumulating evidence links PFOS to oxidative stress, mitochondrial dysfunction, endocrine disruption, neurotoxicity, and immunotoxicity. Critically, PFOS contributes to the development and progression of prostate, breast, and ovarian cancers via mechanisms such as hormonal interference, chronic inflammation, and epigenetic modifications. Epidemiological studies further associate elevated PFOS serum levels with increased cancer risk, particularly in occupationally and environmentally exposed populations. This review brings together the latest knowledge on PFOS emissions, mechanistic toxicity, and cancer-causing potential, highlighting the urgent need for focused research and improved regulatory measures to safeguard public health.
Keywords: breast cancer; carcinogenesis; ovarian cancer; perfluorooctane sulfonate; persistent organic pollutants; prostate cancer; toxicodynamics; toxicokinetic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Cheng J., Huang L., Li Y., Zhang Z., Mu R., Liu C., Hu S., Xiao Y., Xu M. A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water. Processes. 2023;11:2260. doi: 10.3390/pr11082260. - DOI
-
- Li M., Jin Y.-T., Yan J.-F., Liu Z., Feng N.-X., Han W., Huang L.-W., Li Q.-K., Yeung K.-L., Zhou S.-Q., et al. Exploration of perfluorooctane sulfonate degradation properties and mechanism via electron-transfer dominated radical process. Water Res. 2022;215:118259. doi: 10.1016/j.watres.2022.118259. - DOI - PubMed
-
- Li M., Mo C.-H., Luo X., He K.-Y., Yan J.-F., Wu Q., Yu P.-F., Han W., Feng N.-X., Yeung K.L., et al. Exploring key reaction sites and deep degradation mechanism of perfluorooctane sulfonate via peroxymonosulfate activation under electrocoagulation process. Water Res. 2021;207:117849. doi: 10.1016/j.watres.2021.117849. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

