Artificial Intelligence in Thyroid Cytopathology: Diagnostic and Technical Insights
- PMID: 41228318
- PMCID: PMC12610226
- DOI: 10.3390/cancers17213525
Artificial Intelligence in Thyroid Cytopathology: Diagnostic and Technical Insights
Abstract
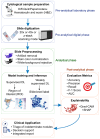
Fine-needle aspiration cytology (FNAC) is the cornerstone of thyroid nodule evaluation, standardized by the Bethesda System. However, indeterminate categories (Bethesda III-IV) remain a major challenge, often leading to unnecessary surgery or delayed molecular testing. Deep learning (DL) has recently emerged as a promising adjunct in thyroid cytopathology, with applications spanning triage support, Bethesda category classification, and integration with molecular data. Yet, routine adoption is limited by preanalytical variability (staining, slide preparation, Z-stack acquisition, scanner heterogeneity), annotation bias, and domain shift, which reduce generalizability across centers. Most studies remain retrospective and single-institution, with limited external validation. This article provides a technical overview of DL in thyroid cytology, emphasizing preanalytical sources of variability, architectural choices, and potential clinical applications. We argue that standardized datasets, multicenter prospective trials, and robust explainability frameworks are essential prerequisites for safe clinical deployment. Looking forward, DL systems are most likely to enter practice as diagnostic co-pilots, Bethesda classifiers, and multimodal risk-stratification tools. With rigorous validation and ethical oversight, these technologies may augment cytopathologists, reduce interobserver variability, and help transform thyroid cytology into a more standardized and data-driven discipline.
Keywords: Bethesda system; artificial intelligence; convolutional neural networks; deep learning; explainable AI; molecular prediction; multimodal models; multiple instance learning; thyroid cytology.
Conflict of interest statement
U.M., Consulting or advisory role (unrelated to the current work): Boehringer Ingelheim, MSD, Roche, Amgen, Lilly, Thermo Fisher Scientific, Diaceutics, Merck, Glaxo Smith Kline, Astra Zeneca; Speakers’ Bureau (unrelated to the current work): Boehringer Ingelheim, Roche, AstraZeneca, MSD, Merck, Amgen, Thermo Fisher Scientific, Diaceutics, Lilly, Glaxo Smith Kline. A.M. has received support from Menarini Group and served on the Speakers’ Bureau for Roche and AstraZeneca. G.C. has received honoraria for speaker engagements from Roche, Seattle Genetics, Novartis, Lilly, Pfizer, Foundation Medicine, NanoString, Samsung, Celltrion, BMS, and MSD; honoraria for consultancy from Roche, Seattle Genetics, and NanoString; honoraria for participation in advisory boards from Roche, Lilly, Pfizer, Foundation Medicine, Samsung, Celltrion, and Mylan; honoraria for writing engagements from Novartis and BMS; and honoraria for participation in the Ellipsis Scientific Affairs Group. He has also received institutional research funding for conducting phase I and II clinical trials from Pfizer, Roche, Novartis, Sanofi, Celgene, Servier, Orion, AstraZeneca, Seattle Genetics, AbbVie, Tesaro, BMS, Merck Serono, Merck Sharp & Dohme, Janssen-Cilag, Philogen, Bayer, Medivation, and Medimmune. K.V. Has received honoraria for speaker bureau from Merck Sharp & Dohme (MSD), Roche, and AstraZeneca; E.G-R. has received advisory fees, honoraria, travel accommodations/expenses, grants, and/or non-financial support from AstraZeneca, Exact Sciences, GSK, Illumina, MSD, Novartis, Roche, and Thermo Fisher Scientific. N.F. has received honoraria for consulting, advisory role, speaker bureau, travel, and/or research grants from Merck Sharp & Dohme (MSD), Merck, Novartis, AstraZeneca, Roche, Menarini Group, Daiichi Sankyo, GlaxoSmithKline (GSK), Gilead, Sysmex, Genomic Health, Veracyte, Sakura, Leica Biosystems, Lilly, Pfizer, ThermoFisher, Abbvie. These companies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and/or in the decision to publish the results. All other authors declare no potential conflicts of interest.
Figures
References
-
- Zamora E.A., Khare S., Cassaro S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2025. Thyroid nodule. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

