Whole Exome Sequencing in Children With Autoimmune Hepatitis Identified Mutations in Genes Involved in the mTORC1 Signaling Pathway
- PMID: 41234702
- PMCID: PMC12605072
- DOI: 10.1016/j.gastha.2025.100808
Whole Exome Sequencing in Children With Autoimmune Hepatitis Identified Mutations in Genes Involved in the mTORC1 Signaling Pathway
Abstract
Background and aims: Autoimmune hepatitis (AIH), a severe immune-mediated liver disease resulting from defective immune tolerance, was thought to be caused by monogenic predisposition with possible external triggers. The development of AIH in monogenic primary immune deficiencies prompted us to search for causative genetic defects in AIH.
Methods: Twenty-two children with AIH were included for whole exome sequencing analysis. Data were analyzed with an in-house software, combined with in silico tools and confirmed by Sanger sequencing. Mechanistic target of rapamycin (mTOR) pathway activation was assessed by phosphoS6-RP expression by fluorescence activated cells sorting.
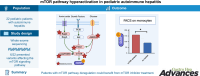
Results: In six children with type 1 or type 2 AIH, seven rare missense candidate variants with damaging predictive scores were identified in five genes involved in the regulation of the mTOR complex 1 (mTORC1) signaling pathway (RRAGC, LAMTOR3, MTOR, TSC2, and PRKAG1). Excess of phosphorylated-S6 ribosomal protein expression both ex vivo by monocytes and in vitro by activated T cells demonstrated an abnormal activation of mTORC1 in five out of six tested patients. Persistent mTORC1 activation in starved activated T cells could be abolished by mTOR inhibitor.
Conclusion: These data showed that pediatric patients with AIH-1 or AIH-2, especially when burdened with poly-autoimmunity, may carry mutations in key partners of the mTORC1 signaling pathway. Moreover, even without identified genetic defect, we observed a deregulation of the mTOR pathway in five out of six tested patients. These results shed new light on the pathogenesis of AIH. Moreover, they suggested that the patients could benefit from specific targeted agents such as mTOR inhibitors.
Keywords: Autoimmune Hepatitis; Genetics Pediatric Auto-Immunity Rapamycin; mTOR Pathway.
© 2025 The Authors.
Figures



References
-
- Mieli-Vergani G., Vergani D., Czaja A.J., et al. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:1–21. - PubMed
-
- Maggiore G., Bernard O., Mosca A., et al. Long-term outcomes of patients with type 1 or 2 autoimmune hepatitis presenting in childhood. J Hepatol. 2023;78:979–988. - PubMed
-
- Terziroli Beretta-Piccoli B., Mieli-Vergani G., Vergani D. Serology in autoimmune hepatitis: a clinical-practice approach. Eur J Intern Med. 2018;48:35–43. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

