Divergent Effects of Cytomegalovirus and Rheumatoid Arthritis on Senescent CD4+ T Cells
- PMID: 41235706
- PMCID: PMC12616766
- DOI: 10.1002/eji.70093
Divergent Effects of Cytomegalovirus and Rheumatoid Arthritis on Senescent CD4+ T Cells
Abstract
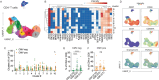
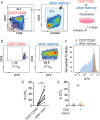
Chronic antigen exposure drives CD4⁺ T cell senescence, yet how autoimmunity and persistent viral infections differentially shape T cell differentiation and function remains unclear. Using cytomegalovirus (CMV) and rheumatoid arthritis (RA) as models of chronic immune activation, we performed high-dimensional mass cytometry and functional assays to define their impact on CD4⁺ T cells. In CMV-seropositive individuals, CD27-CD28- CD4⁺ T cells were abundant and exhibited a predominantly cytotoxic, nonproliferative phenotype. Only a minor fraction was CMV-reactive, suggesting that bystander-driven differentiation contributes to this subset. In the absence of CMV, senescent CD4⁺ T cells were infrequent and phenotypically distinct, though they still exhibited low proliferative capacity. EBV and HSV did not independently increase CD27-CD28- CD4⁺ T cell frequency. Similarly, RA had little effect on their abundance but instead tuned the functional quality of senescent cells. In CMV-seropositive RA patients, senescent CD4⁺ T cells produced less pro-inflammatory cytokines and showed impaired cytotoxic degranulation. Central memory CD4⁺ and CD27-CD28- CD8⁺ T cell functions were preserved, with no evidence for CMV reactivation, suggesting maintained viral control by unaffected T cell responses. These findings highlight distinct, nonredundant effects of CMV and RA on CD4⁺ T cell senescence and reveal RA-specific functional defects in senescent CD4⁺ T cells.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

