Preclinical Evaluation of a Pilocarpine-(R)-Lipoic Acid Eye Drop for Presbyopia
- PMID: 41235858
- PMCID: PMC12629135
- DOI: 10.1167/tvst.14.11.17
Preclinical Evaluation of a Pilocarpine-(R)-Lipoic Acid Eye Drop for Presbyopia
Abstract
Purpose: Presbyopia is a progressive, age-related loss of near vision. Although current therapies offer symptomatic relief, they fail to target the underlying pathology. These studies investigated a novel dual-mechanism eye drop, CLX-162 (pilocarpine lipoate salt), focusing on three key characteristics: (1) tolerability, (2) pharmacokinetics and ocular tissue penetration, and (3) chemical stability within a dual-chamber delivery system.
Methods: Tolerability and pharmacokinetic studies involved administering CLX-162 and lipoic acid choline ester (LACE) ophthalmic formulations to New Zealand White rabbits. Investigators assessed ocular tolerability using the Draize scoring system and evaluated pharmacokinetics by collecting and analyzing ocular tissues. A third study evaluated CLX-162 stability by storing it in a dual-chamber system under varying conditions and analyzing the drug substance and reconstituted product.
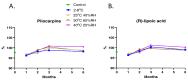
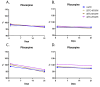
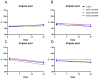
Results: CLX-162 demonstrated superior ocular tolerability compared to LACE, with no corneal, iridial, or conjunctival effects observed. It induced transient pupillary constriction, whereas LACE caused mild redness and discharge. Pharmacokinetic analysis showed that CLX-162 achieved significantly higher and longer lasting (R)-lipoic acid levels in the aqueous humor and lens than LACE. Pilocarpine remained detectable for up to 8 hours. Stability studies confirmed that CLX-162 retained potency for 6 months in the dual-chamber container, with pilocarpine and (R)-lipoic acid levels within 95% to 100%. After reconstitution, it remained stable for 21 days.
Conclusions: These preclinical studies demonstrated the stability, penetrability, and safety of CLX-162 dispensed in a dual-chamber, supporting progression to clinical trials.
Translational relevance: The dual-mechanism design of CLX-162 addresses the oxidative stress-driven lens changes underlying presbyopia, bridging preclinical findings to future patient care.
Conflict of interest statement
Disclosure:
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

