Process evaluation findings contradict RCT results of the IBD-BOOST digital self-management intervention for fatigue, pain and faecal urgency in inflammatory bowel disease: A mixed methods study of patient perspectives
- PMID: 41236397
- PMCID: PMC12617385
- DOI: 10.1111/bjhp.70035
Process evaluation findings contradict RCT results of the IBD-BOOST digital self-management intervention for fatigue, pain and faecal urgency in inflammatory bowel disease: A mixed methods study of patient perspectives
Abstract
Purpose: This parallel process evaluation examined the implementation of a randomised controlled trial (RCT) of IBD-BOOST-a digital, interactive, facilitator-supported self-management intervention targeting fatigue, pain and urgency/faecal incontinence in individuals with inflammatory bowel disease (IBD). The RCT, involving 780 participants, compared IBD-BOOST with usual care but found no significant differences in quality of life (QoL) or symptom relief at six months post-randomisation.
Methods: A concurrent mixed methods design was employed. Qualitative data were gathered through semi-structured interviews; quantitative data were derived from the intervention platform's built-in analytics. Qualitative data were analysed using narrative thematic and framework analysis; quantitative data were examined using descriptive statistics.
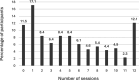
Results: Interviews were conducted with participants pre- (n = 30) and post-intervention (n = 28). At baseline, participants highlighted a need for improved education and support targeting fatigue, pain and urgency/faecal incontinence, expressing a preference for digital delivery due to its flexibility. Post-intervention, treatment group interviewees reported high satisfaction with the intervention's content and structure, with many continuing to use the strategies learned, reporting enhanced symptom management and QoL. However, quantitative data indicated low adherence. Control group interviewees expressed disappointment with their allocation but anticipated benefits from deferred access to the intervention.
Conclusions: Although the RCT found no statistically significant effect of IBD-BOOST on primary outcomes, the process evaluation results revealed perceived benefits in symptom understanding and developing new management strategies. The intervention was well-received, and patients reported improvements in QoL. There was strong patient support for the IBD-BOOST intervention to be freely available to all individuals with IBD.
Keywords: digital intervention; inflammatory bowel disease; process evaluation; randomised controlled trial; symptom management.
© 2025 The Author(s). British Journal of Health Psychology published by John Wiley & Sons Ltd on behalf of British Psychological Society.
Conflict of interest statement
WC‐D: Speaker fees from Dr. Falk, Pharmacosmos and research funding from Bristol Myers Squibb and Crohn's and Colitis UK; LD: Speaker fees from Janssen, Tillotts Pharma UK, Ferring Pharmaceuticals; research funding from Janssen and Takeda; CN: Speaker fees from Medscape, Merck Pharmaceutical; Tillotts Pharma UK, Lilly. Pfizer advisory board; RM‐M: A beneficiary of a licence agreement signed between King's College London and Mahana Therapeutics for a digital cognitive behavioural therapy for an irritable bowel syndrome product. RMM receives personal fees from Mahana Therapeutics for scientific advisory work and from other universities and hospital trusts for cognitive behavioural therapy training in irritable bowel syndrome. Other co‐authors declare no competing interests.
Figures
References
-
- Bellg, A. J. , Borrelli, B. , Resnick, B. , Hecht, J. , Minicucci, D. S. , Ory, M. , Ogedegbe, G. , Orwing, D. , Ernst, D. , & Czajkowski, S. (2004). Enhancing treatment fidelity in health behaviour change studies: Best practices and recommendations from the NIH behaviour change consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 - DOI - PubMed
-
- Braun, V. , & Clarke, V. (2022). Conceptual and design thinking for thematic analysis. Qualitative Psychology, 9(1), 3–26. 10.1037/qup0000196 - DOI
-
- Burisch, J. , Zhao, M. , Odes, S. , De Cruz, P. , Vermeire, S. , Bernstein, C. N. , Kaplan, G. G. , Duricova, D. , Greenberg, D. , Melberg, H. O. , Watanabe, M. , Ahn, H. S. , Targownik, L. , Pittet, V. E. H. , Annese, V. , Park, K. T. , Katsanos, K. H. , Høivik, M. L. , Krznaric, Z. , … Munkholm, P. (2023). The cost of inflammatory bowel disease in high‐income settings: A Lancet Gastroenterology & Hepatology Commission. The Lancet Gastroenterology & Hepatology, 8(5), 458–492. 10.1016/S2468-1253(23)00003-1 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical