High-Dose vs. Standard-Dose Influenza Vaccine in Older Patients With Hypertension: A Post Hoc Analysis of DANFLU-1
- PMID: 41238377
- PMCID: PMC12618134
- DOI: 10.1111/jch.70177
High-Dose vs. Standard-Dose Influenza Vaccine in Older Patients With Hypertension: A Post Hoc Analysis of DANFLU-1
Abstract
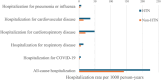
Patients with hypertension (HTN) face an increased risk of complications and mortality from influenza; a risk that may be modified by influenza vaccination. There is limited evidence on the effectiveness of high-dose (HD-IIV) compared with standard-dose (SD-IIV) inactivated influenza vaccines in hypertensive individuals. This study, a post hoc analysis of DANFLU-1, a pragmatic, and open-label, individually randomized trial of HD-IIV vs. SD-IIV conducted during the 2021-2022 influenza season among participants aged 65-79 years. Prespecified outcomes in DANFLU-1 included hospitalization for influenza or pneumonia, cardiovascular, cardiorespiratory, and respiratory hospitalizations, all-cause hospitalization, and all-cause mortality. Outcomes were analyzed as both time-to-first and recurrent events. DANFLU-1 randomized 12 477 participants randomized to HD-IIV or SD-IIV, of these 6469 (51.9%) had a history of HTN. HD-IIV was associated with lower hazards for hospitalizations for pneumonia or influenza, respiratory disease, and all-cause mortality compared with SD-IIV and these associations were consistent regardless of HTN status (pinteraction = 0.09, = 0.09, and = 0.59, respectively). HD-IIV was associated with lower incidence rates of recurrent hospitalizations for pneumonia or influenza and all-cause hospitalizations compared with SD-IIV, irrespective of HTN status (pinteraction = 0.09 and = 0.75, respectively). There was evidence of potential effect modification of HD-IIV vs. SD-IIV in relation to recurrent respiratory hospitalizations, where the relative effect may be greater among those without vs. with HTN (pinteraction = 0.04). In conclusion, this post hoc analysis of a large-scale pragmatic trial, HD-IIV was associated with lower risk of clinical outcomes, including hospitalizations for pneumonia or influenza, all-cause mortality, and all-cause hospitalizations irrespective of the presence of HTN. Trial Registration: ClinicalTrials.gov identifier: NCT05048589.
© 2025 The Author(s). The Journal of Clinical Hypertension published by Wiley Periodicals LLC.
Conflict of interest statement
A.F.L. has received speaker honoraria from Novo Nordisk. S.S., M.M.L., J.N., and R.C.H. are full‐time employees of Sanofi and may own shares and/or stock options in the company. C.S.L. is chief physician at Danske Lægers Vaccinations Service, part of the European LifeCare Group, and has received speaker fees and served on advisory boards for GSK, MSD, Pfizer, Takeda, and Valneva. B.L.C. has received consulting fees from Amgen, Cardurion, Corvia, Myokardia, and Novartis. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi, Theracos, and US2. M.J.L. has received institutional research grants from Sanofi, GSK, Regeneron, Moderna, Novartis, and Apollo Rx L.K. has received speaker fees from Novo Nordisk, Novartis, AstraZeneca, Boehringer Ingelheim, and Bayer. T.B.‐S. has served as steering committee member of the Amgen financed GALACTIC‐HF trial, the Boehringer Ingelheim financed SHARP3 trial, and the Boston Scientific financed LUX‐Dx TRENDS trial, served on advisory boards for Sanofi, GSK and Amgen, received speaker honoraria from Bayer, GSK, Novartis, Novo Nordisk and Sanofi, and received research grants from GE Healthcare, AstraZeneca, Novo Nordisk and Sanofi. All other authors have no conflicts of interest to declare.
Figures


References
-
- Fryar C. D., Ostchega Y., Hales C. M., Zhang G., and Kruszon‐Moran D., “Key Findings Data from the National Health and Nutrition Examination Survey.” (2015), https://www.cdc.gov/nchs/data/databriefs/db289_table.pdf#2.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

