Automated reporting of primaquine dose efficacy, tolerability and safety for Plasmodium vivax malaria using a systematic review and individual patient data meta-analysis
- PMID: 41239453
- PMCID: PMC12619205
- DOI: 10.1186/s12936-025-05642-w
Automated reporting of primaquine dose efficacy, tolerability and safety for Plasmodium vivax malaria using a systematic review and individual patient data meta-analysis
Abstract
Background: The antirelapse efficacy of primaquine is related to the total dose administered, whereas the risks of haemolysis and gastrointestinal intolerance are associated with the daily dose administered. National Malaria Control Programmes require local information on efficacy, tolerability and safety to optimize antimalarial treatment policies for Plasmodium vivax malaria control and elimination efforts.
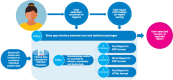
Methods: A living systematic review identified efficacy studies of uncomplicated P. vivax malaria including patients treated with daily primaquine regimens, published since January 1, 2000. Available data were pooled and an R Shiny app was developed to integrate statistical analyses performed using R and Stata that assessed the impact of primaquine mg/kg dose on efficacy, hematological safety and gastrointestinal tolerability.
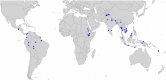
Results: As of January 16, 2025, a total of 9,270 individual patient data records from 41 studies have been collated into the standardized repository. Open-access automated reports were generated for user-selected countries or regions to investigate location specific effects of primaquine dose on efficacy, safety and tolerability. The reports include visual and tabular displays of the outcomes.
Conclusions: These automated reports leverage a large database to provide accessible data for national and regional policy makers and researchers to assess the clinical consequences of different primaquine regimens in different endemic settings. The reports will inform local and regional policy decisions and research priorities in vivax-endemic areas.
Keywords: Plasmodium vivax; Automated report; Dose; Efficacy; Haemolysis; Malaria; Primaquine; Recurrence; Relapse; Tolerability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Shared data were obtained according to ethical approvals from the country of origin and original study and were shared in a controlled framework with specific restrictions on their use. The data are pseudonymised and unable to be linked to individuals. As such, this analysis did not require additional ethical approval according to the guidelines of the Oxford Central University Research Ethics Committee. Consent for publication: Not applicable. Competing interests: JAG and GCKWK are former employees of GSK and hold shares in GSK and AstraZeneca. GCKWK reports travel support from AstraZeneca. JKB reports institutional research funding from MMV, GSK, the Wellcome Trust, and Sanaria; participation on the US National Institute of Health Data Safety Monitoring Board; and membership of the Editorial Board of Travel Medicine and Infectious Diseases and the Guidelines Development Group for Malaria Control and Elimination, Global Malaria Program, WHO. RJC, RNP and JKB report contributions to Up-to-Date. NMA reports membership of the Writing Group for the Australian national Malaria Treatment Guidelines: Therapeutic Guidelines, 2025. All other authors declare no conflicts of interest.
Figures
References
-
- Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12:e1001891. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials