Impact of moisture on microbial decomposition phenotypes and enzyme dynamics
- PMID: 41239974
- PMCID: PMC12649754
- DOI: 10.1093/ismejo/wraf250
Impact of moisture on microbial decomposition phenotypes and enzyme dynamics
Abstract
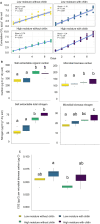
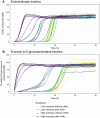
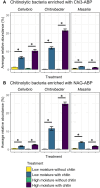
Soil organic matter decomposition is a complex process reflecting microbial composition and environmental conditions. Moisture can modulate the connectivity and interactions of microbes. Due to heterogeneity, a deeper understanding of the influence of soil moisture on the dynamics of organic matter decomposition and resultant phenotypes remains a challenge. Soils from a long-term field experiment exposed to high and low moisture treatments were incubated in the laboratory to investigate organic matter decomposition using chitin as a model substrate. By combining enzymatic assays, biomass measurements, and microbial enrichment via activity-based probes, we determined the microbial functional response to chitin amendments and field moisture treatments at both the community and cell scales. Chitinolytic activities showed significant responses to the amendment of chitin, independent of differences in field moisture treatments. However, for other measurements of carbon metabolism and cellular functions, soils from high moisture field treatments had greater potential enzyme activity than soils from low moisture field treatments. A cell tagging approach was used to enrich and quantify bacterial taxa that are actively producing chitin-degrading enzymes. By integrating organism, community, and soil core measurements we show that (i) a small subset of taxa compose the majority (>50%) of chitinase production despite broad functional redundancy, (ii) the identity of key chitin degraders varies with moisture level, and (iii) extracellular enzymes that are not cell-associated account for most potential chitinase activity measured in field soil.
Keywords: activity-based probes; carbon use efficiency; chitin; soil microbiome.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schimel JP. Life in dry soils: effects of drought on soil microbial communities and processes. Annu Rev Ecol Evol Syst 2018;49:409–32. 10.1146/annurev-ecolsys-110617-062614 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

