Evaluation of self-mediated alternatives for risk testing education and return of results (eSMARTER) study: A randomized study of methods for returning APOE and pTau-217 results
- PMID: 41250770
- PMCID: PMC12620075
- DOI: 10.1002/trc2.70177
Evaluation of self-mediated alternatives for risk testing education and return of results (eSMARTER) study: A randomized study of methods for returning APOE and pTau-217 results
Abstract
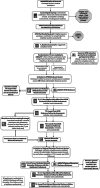
Introduction: Disclosing Alzheimer's disease (AD) genetic and biomarker information is becoming increasingly common but often resource-intensive, requiring a clinician to return results. As results become relevant for clinical care and more people want to learn their results, scalable approaches to disclosure are needed. Here, we describe the design of a decentralized, randomized, non-inferiority clinical trial (eSMARTER) to evaluate self-directed scalable digital methods for returning APOE and plasma pTau-217 results.
Methods: Participants (n ≈ 600) aged 60-80 years are randomized to receive their apolipoprotein E (APOE) and pTau-217 results via either a clinician-mediated telehealth videoconference (usual care) or a self-mediated eHealth platform. The primary hypothesis is that self-mediated APOE disclosure will result in non-inferior outcomes compared to clinician-mediated disclosure. The three primary outcomes are changes in anxiety, AD-related knowledge, and disease-specific distress. For the three primary analyses, we will apply non-inferiority tests to examine if self-mediated disclosure provides equivalent or improved outcomes compared to usual care. Our secondary hypothesis is that learning APOE and pTau-217 results will affect participants' mood, self-perception of disease risk, and quality of life. We hypothesize participants who learn results indicating more risk will report increased, but not clinically significant psychological distress and increased disease risk. We hypothesize there will not be changes in quality of life. We will examine change scores of the validated mood, self-perception of disease risk, and quality of life measures from pre- to post-disclosure.
Results: Data collection is ongoing. Results will allow for non-inferiority comparisons between clinician- and self-mediated disclosure of APOE results and initial characterization of the impact of learning pTau-217 results.
Discussion: The eSMARTER Study represents an important step towards meeting expected increases in demand for learning AD genetic and biomarker information. The digital platforms developed for this study will be made available to support clinicians and investigators returning genetic and/or biomarker results.
Highlights: Novel, scalable design for self-mediated disclosure of APOE and pTau-217.Non-inferiority trial examining self-mediated versus clinician-mediated disclosure.All materials, methods, data, and samples will be shared publicly.
Keywords: APOE; digital methods; non‐inferiority trial; pTau‐217; return of results; self‐disclosure.
© 2025 The Author(s). Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Jessica B. Langbaum reports receiving consulting fees from Denovo Biopharma and Premiere Inc. Emily A. Largent reports receiving consulting fees from Novartis. Eric M. Reiman is a compensated scientific advisor for Alzheon, Cognition Therapeutics, Denali Therapeutics, Enigma, Jocanta, Retromer Therapeutics, and Vaxxinity. He is a co‐founder, advisor, and shareholder in ALZPath. Jason Karlawish reports receiving consultation fees from Biogen and Darmiyan. The rest of the authors have no conflicts of interest to report. The rest of the authors have no relevant disclosures to report. Author disclosures are available in the Supporting Information.
References
-
- Clark LR, Chin NA, Erickson CM, et al. Impact of learning amyloid PET results on test‐related distress and concern about dementia in a cognitively unimpaired observational cohort. Alzheimers Dement. 2023;19(S19):e078746. doi: 10.1002/alz.078746 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous