Optimization of Prodiginines as Single-Dose Curative Antimalarials
- PMID: 41253140
- PMCID: PMC12649828
- DOI: 10.1021/acs.jmedchem.5c02523
Optimization of Prodiginines as Single-Dose Curative Antimalarials
Abstract
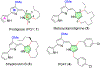
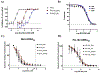
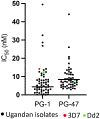
The emergence of drug-resistant malaria underscores the urgent need for novel, well-tolerated antimalarials with multistage activity and distinct mechanisms of action. Here, we report the design and synthesis of 54 new prodiginine (PG) analogs through systematic modification of the B and C rings of the core scaffold. This effort led to the identification of lead compound PG102 (6), which demonstrated curative oral efficacy in the erythrocytic Plasmodium yoelii murine model at both 25 mg/kg × 4 days and 80 mg/kg × 1 day dosing regimens. PG102 also provided protection against liver-stage Plasmodium berghei sporozoite-induced infection at 20 mg/kg × 3 days and retained high potency against artemisinin-resistant Plasmodium falciparum strains. Profiling of PG102 demonstrated a medium parasite-killing profile. Notably, PG102 exhibited low potential for genotoxicity and cardiotoxicity. This study provides the first demonstration of robust single-dose antimalarial efficacy within the prodiginine class, paving the way for the development of next-generation antimalarial agents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
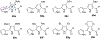

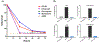


References
-
- WHO World Malaria Report; WHO, 2024.
-
- Arya A; Foko LPK; Chaudhry S; Sharma A; Singh V Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions - India and sub-Saharan Africa. Int. J. Parasitol. Drugs Drug Resist 2021, 15, 43–56. - PMC - PubMed
-
- Uwimana A; Legrand E; Stokes BH; Ndikumana JM; Warsame M; Umulisa N; Ngamije D; Munyaneza T; Mazarati JB; Munguti K; Campagne P; Criscuolo A; Ariey F; Murindahabi M; Ringwald P; Fidock DA; Mbituyumuremyi A; Menard D Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med 2020, 26, 1602–1608. - PMC - PubMed
-
- Rosenthal PJ; Asua V; Conrad MD Emergence, transmission dynamics and mechanisms of artemisinin partial resistance in malaria parasites in Africa. Nat. Rev. Microbiol 2024, 22, 373–384. - PubMed
-
- Ishengoma DS; Gosling R; Martinez-Vega R; Beshir KB; Bailey JA; Chimumbwa J; Sutherland C; Conrad MD; Tadesse FG; Juliano JJ; Kamya MR; Mbacham WF; Menard D; Rosenthal PJ; Raman J; Tatarsky A; Tessema SK; Fidock DA; Djimde AA Urgent action is needed to confront artemisinin partial resistance in African malaria parasites. Nat. Med 2024, 30, 1807–1808. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

