Proteomic Profiling Reveals Mitochondrial Dysregulation in Rapidly Progressive Alzheimer's: Role of DLDH in Amyloid Beta Aggregation
- PMID: 41254340
- PMCID: PMC12627125
- DOI: 10.1007/s12035-025-05327-0
Proteomic Profiling Reveals Mitochondrial Dysregulation in Rapidly Progressive Alzheimer's: Role of DLDH in Amyloid Beta Aggregation
Abstract
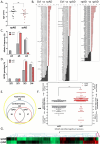
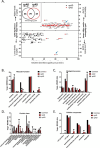
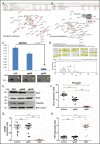
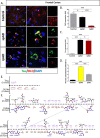
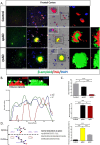
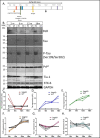
Alzheimer's disease (AD) is presented as multiple clinical variants depending upon the rate of progression and familial background; however, the exact molecular mechanisms associated with these subtypes and their treatments are yet to be understood. The current study is based on a global proteome analysis of brain samples from patients (n = 38) with rapidly progressive AD (rpAD-survival time < 3 years), sporadic AD (spAD-survival time of 8-10 years), and healthy controls. Proteome analysis revealed a differential regulation of 79 proteins and highlighted the dysregulation of mitochondrial machinery and glucose metabolism in rpAD. Dihydrolipoamide dehydrogenase (DLDH), a mitochondrial oxidoreductase, showed a significant reduction and delocalization in rpAD. In vitro analysis revealed a potential role of DLDH in the aggregation of amyloid beta. Rapid progression in AD may be influenced by the energy homeostasis and redox dysfunction linked with the DLDH.
Keywords: Alzheimer’s disease (AD); DLDH; Metabolic networks; Metabolism; Post-translational modification; Proteomics; Rapidly progressive Alzheimer’s disease (rpAD).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Alzheimer’s Association, 2023 Alzheimer’s disease facts and figures. Alzheimer’s & Dement 2023;19(4):1598–695 - PubMed
-
- Schmidt C, Redyk K, Meissner B, Krack L, Von Ahsen N, Roeber S, Kretzschmar H, Zerr I (2010) Clinical features of rapidly progressive Alzheimer’s disease. Dement Geriatr Cogn Disord 29(4):371–378 - PubMed
-
- Schmidt C, Haïk S, Satoh K, Rábano A, Martinezmartin P, Roeber S, Brandel JP, Calero-Lara M et al (2012) Rapidly progressive Alzheimer’s disease: a multicenter update. J Alzheimers Dis 30(4):751–756 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

