Light and Alternating Temperatures Release Seed Dormancy in the Invasive Dipsacus fullonum L. Through ROS Homeostasis and ABA Regulation
- PMID: 41255306
- PMCID: PMC12628119
- DOI: 10.1111/ppl.70642
Light and Alternating Temperatures Release Seed Dormancy in the Invasive Dipsacus fullonum L. Through ROS Homeostasis and ABA Regulation
Abstract
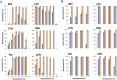
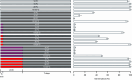
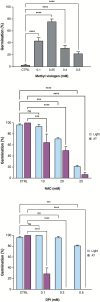
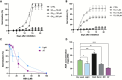
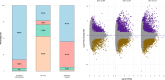
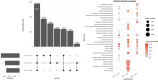
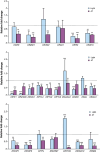
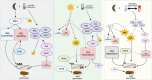
Seeds have developed mechanisms to perceive environmental signals, such as light and temperature, which govern germination and enhance the chance of seedling establishment. This study examined the foundations of light and temperature sensitivity in the seed dormancy release of a common weed, Dipsacus fullonum. By screening six accessions from two different regions, we identified two unique germination behaviors: one sensitive to environmental stimuli and one neutral to them. In the sensitive accession, ABA is crucial for regulating dormancy release, as it accumulates in seeds subjected to darkness, while it decreases under other conditions. We observed a rise of reactive oxygen species (ROS) under conditions that stimulate germination and highlighted that their presence enhanced germination even in the absence of light. This study employed a long-read RNA-seq technology to examine the regulation of key genes associated with germination. We identified the essential nodes in this process: DfPIF1, which maintains the dormancy state in darkness at constant temperatures mainly by promoting ABA biosynthesis and signaling, and antioxidant enzymatic machinery, DfMSD1, DfCSD2, DfAPX, and DfPRX1, whose activity regulates ROS homeostasis, promoting or inhibiting germination. This study provides novel mechanisms that regulate seed germination in weeds, specifically involving ABA regulation and ROS in response to environmental stimuli.
Keywords: PIF1; ROS scavengers; common teasel; environmental cues; histone deacetylase; long‐reads RNA‐seq; seed germination; weeds.
© 2025 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Conflict of interest statement
In this manuscript no generative AI has been employed.
The authors declare no conflicts of interest.
Figures


References
-
- Arana, M. V. , Tognacca R. S., Estravis‐Barcalá M., Sánchez R. A., and Botto J. F.. 2017. “Physiological and Molecular Mechanisms Underlying the Integration of Light and Temperature Cues in <styled-content style="fixed-case"> Arabidopsis thaliana </styled-content> Seeds.” Plant, Cell & Environment 40: 3113–3121. - PubMed
-
- Bailly, C. 2004. “Active Oxygen Species and Antioxidants in Seed Biology.” Seed Science Research 14: 93–107.
-
- Bailly, C. 2019. “The Signalling Role of ROS in the Regulation of Seed Germination and Dormancy.” Biochemical Journal 476: 3019–3032. - PubMed
-
- Bailly, C. , El‐Maarouf‐Bouteau H., and Corbineau F.. 2008. “From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology.” Comptes Rendus Biologies 331: 806–814. - PubMed
-
- Baud, S. , Corso M., Debeaujon I., et al. 2022. “Recent Progress in Molecular Genetics and Omics‐Driven Research in Seed Biology.” Comptes Rendus Biologies 345: 61–110. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

