This is a preprint.
Retrograde mitochondrial transport regulates mitochondrial biogenesis in zebrafish neurons
- PMID: 41256681
- PMCID: PMC12621953
- DOI: 10.1101/2025.09.29.679307
Retrograde mitochondrial transport regulates mitochondrial biogenesis in zebrafish neurons
Abstract
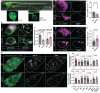
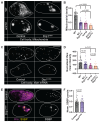
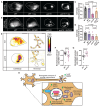
To maintain a healthy mitochondrial population in a long-lived cell like a neuron, mitochondria must be continuously replenished through the process of mitochondrial biogenesis. Because the majority of mitochondrial proteins are nuclear encoded, mitochondrial biogenesis requires nuclear sensing of mitochondrial population health and function. This can be a challenge in a large, compartmentalized cell like a neuron in which a large portion of the mitochondrial population is in neuronal compartments far from the nucleus. Using in vivo assessments of mitochondrial biogenesis in zebrafish neurons, we determined that mitochondrial transport between distal axonal compartments and the cell body is required for sustained mitochondrial biogenesis. Estrogen-related receptor transcriptional activation links transport with mitochondrial gene expression. Together, our data support a role for retrograde feedback between axonal mitochondria and the nucleus for regulation of mitochondrial biogenesis in neurons.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Jäger S., Handschin C., St.-Pierre J., and Spiegelman B.M. (2007). AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proceedings of the National Academy of Sciences 104, 12017–12022. 10.1073/pnas.0705070104. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
