Probiotics Supplementation in Tuberculosis: A Scoping Review
- PMID: 41256827
- PMCID: PMC12623083
- DOI: 10.1155/sci5/6926727
Probiotics Supplementation in Tuberculosis: A Scoping Review
Abstract
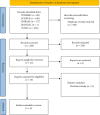
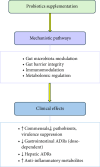
This scoping review aims to synthesize the current clinical evidence on probiotics used in tuberculosis (TB). Systematic literature searches were conducted across PubMed, Scopus, and Embase databases to identify all studies using probiotics in TB. A total of six studies conducted between 2016 and 2023 were found and included in this review. All the studies incorporated probiotics supplementation not beyond the intensive phase of antitubercular therapy (ATT), ranging from three to eight weeks. Five out of six included studies focused on pulmonary TB. Probiotics alleviate adverse gastrointestinal and hepatic drug reactions, modulate gut microbiota, enhance barrier function, and influence immune responses. Therefore, probiotics are a potential adjunct therapy during the intensive phase of ATT. However, their long-term effects remain unexplored, highlighting the future research scope for well-designed longitudinal studies to explore their sustained benefits.
Keywords: antitubercular therapy; probiotics; tuberculosis.
Copyright © 2025 Tejaswini Baral et al. Scientifica published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Global Tuberculosis Report. World Health Organization; 2024. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... .
-
- Jan T., Negi R., Sharma B., et al. Diversity, Distribution and Role of Probiotics for Human Health: Current Research and Future Challenges. Biocatalysis and Agricultural Biotechnology . 2023;53:p. 102889. doi: 10.1016/j.bcab.2023.102889. - DOI
Publication types
LinkOut - more resources
Full Text Sources