A skin-permeable polymer for non-invasive transdermal insulin delivery
- PMID: 41261125
- PMCID: PMC12695667
- DOI: 10.1038/s41586-025-09729-x
A skin-permeable polymer for non-invasive transdermal insulin delivery
Abstract
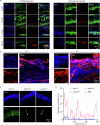
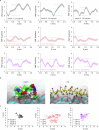
Non-invasive skin permeation is widely used for convenient transdermal delivery of small-molecule therapeutics (less than 500 Da) with appropriate hydrophobicities1. However, it has long been deemed infeasible for large molecules-particularly polymers, proteins and peptides2,3-due to the formidable barrier posed by the skin structure. Here we show that the fast skin-permeable polyzwitterion poly[2-(N-oxide-N,N-dimethylamino)ethyl methacrylate] (OP) can efficiently penetrate the stratum corneum, viable epidermis and dermis into circulation. OP is protonated to be cationic and is therefore enriched in the acidic sebum and paracellular stratum corneum lipids containing fatty acids, and subsequently diffuses through the intercorneocyte lipid lamella. Beneath the stratum corneum, at the normal physiological pH, OP becomes a neutral polyzwitterion, 'hopping' on cell membranes, enabling its efficient migration through the epidermis and dermis and ultimately entering dermal lymphatic vessels and systemic circulation. As a result, OP-conjugated insulin efficiently permeates through the skin into the blood circulation; transdermal administration of OP-conjugated insulin at a dose of 116 U kg-1 into mice with type 1 diabetes quickly lowers their blood glucose levels to the normal range, and a transdermal dose of 29 U kg-1 normalizes the blood glucose levels of diabetic minipigs. Thus, the skin-permeable polymer may enable non-invasive transdermal delivery of insulin, relieving patients with diabetes from subcutaneous injections and potentially facilitating patient-friendly use of other protein- and peptide-based therapeutics through transdermal delivery.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Muhammad, A. M. et al. Skin-penetrating peptides (SKPs): enhancing skin permeation for transdermal delivery of pharmaceuticals and cosmetic compounds. Int. J. Pharm.672, 125339 (2025). - PubMed
-
- Roberts, M. S. et al. Topical drug delivery: history, percutaneous absorption, and product development. Adv. Drug Deliv. Rev.177, 113929 (2021). - PubMed
-
- Singh Malik, D., Mital, N. & Kaur, G. Topical drug delivery systems: a patent review. Exp. Opin. Ther. Pat.26, 213–228 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

