Nitric oxide-releasing PHEMA/polysilsesquioxane photocrosslinked hybrids
- PMID: 41262760
- PMCID: PMC12624852
- DOI: 10.1039/d5ra07870a
Nitric oxide-releasing PHEMA/polysilsesquioxane photocrosslinked hybrids
Abstract
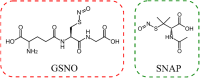
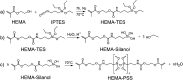
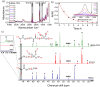
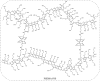
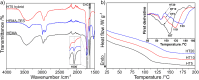
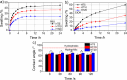
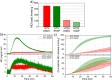
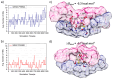
Polymeric materials capable of releasing nitric oxide (NO) locally have potential uses in various biomedical applications. One of the main challenges in this field is obtaining materials that allow the modulation of NO release rates through the incorporation of different NO donor molecules. Herein, we describe the synthesis of materials composed of poly(2-hydroxyethyl methacrylate) (PHEMA) crosslinked by a polysilsesquioxane (PSS) network through sol-gel polymerization and photocrosslinking. Increasing the PSS content from 5 to 20 wt% led to an increase in the glass transition temperature from 107 °C to 133 °C. Swelling studies in phosphate buffer saline solution and ethanol revealed that higher siloxane content reduced the solvent uptake of the hybrids, while surface contact angle measurements confirmed that all compositions remained hydrophilic (60-70°). These hybrids enabled, for the first time, the incorporation of two structurally distinct NO donors, hydrophilic S-nitrosoglutathione (GSNO) and hydrophobic S-nitroso-N-acetyl-dl-penicillamine (SNAP), via absorption from aqueous and ethanolic solutions, respectively. Computational modeling showed that GSNO forms multiple hydrogen bonds with PHEMA hydroxyl groups, while SNAP interacts hydrophobically with its methyl groups. Real-time NO measurements showed that SNAP spontaneously releases NO at a flow rate 2 to 10 times higher than that of GSNO in the first 30 min after hydration of the hybrids, likely due to its weaker intermolecular interactions and higher mobility upon hydration. The hydrophilic nature of the hybrids, tunable NO release, and lack of cytotoxicity toward cultured endothelial cells position them as promising candidates for the manufacturing of antithrombotic blood-contacting medical devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Pandey S. and Mishra S. B., in Nanomedicine for Drug Delivery and Therapeutics, Wiley, 2013, pp. 135–161
-
- Faustini M. Nicole L. Ruiz-Hitzky E. Sanchez C. Adv. Funct. Mater. 2018;28:1704158.
-
- Sanchez C. de G. J. Soler-Illia A. A. Ribot F. Lalot T. Mayer C. R. Cabuil V. Chem. Mater. 2001;13:3061–3083.
-
- Park W. Shin H. Choi B. Rhim W.-K. Na K. Han D. K. Prog. Mater. Sci. 2020;114:100686.
-
- Mir S. H. Nagahara L. A. Thundat T. Mokarian-Tabari P. Furukawa H. Khosla A. J. Electrochem. Soc. 2018;165:B3137–B3156.
LinkOut - more resources
Full Text Sources

