Implantable bioelectronics for gut electrophysiology
- PMID: 41266383
- PMCID: PMC12635400
- DOI: 10.1038/s41467-025-65473-w
Implantable bioelectronics for gut electrophysiology
Abstract
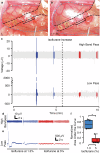
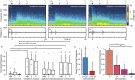
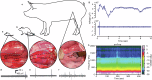
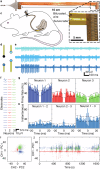
A major regulator of gastrointestinal physiology is the enteric nervous system. This division of the autonomic nervous system is unique in its extensiveness, with neurons distributed from the esophagus to the rectum, and its capability for local information processing. However, the constant motion of the gut, arising from its relative movements in the peritoneal cavity and the peristaltic movements associated with gut motility, as well as the sparse distribution of the neurons constituting the enteric nervous system, has made access and analysis exceedingly challenging. Here, we present the construction and validation of a bioelectronic implant for accessing neural information from the distal colon. Our bioelectronic monitoring system demonstrates real-time electrophysiological recording in response to chemical and mechanical distension under anesthesia and to feeding and stress in freely-moving animals. Direct access to the communication pathways of the enteric nervous system paves the way for neuromodulation strategies targeting the gut-brain axis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

