A signal-seeking phase 2 study of tremelimumab in advanced cancers with high tumour mutational burden
- PMID: 41272153
- PMCID: PMC12639129
- DOI: 10.1038/s41698-025-01156-5
A signal-seeking phase 2 study of tremelimumab in advanced cancers with high tumour mutational burden
Abstract
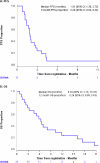
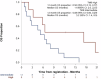
This single-arm phase II trial (ACTRN12620000918921) evaluated the clinical activity of tremelimumab (10 mg/kg intravenously every 4 weeks for 6 cycles) in advanced cancers with a high tumour mutational burden (TMB), defined as TMB > 10 mutations/megabase (mut/Mb) on standard platforms (TSO500 panel, F1CDx), or >20 mut/Mb on the TST170 panel. The primary objective was 6-month progression-free survival rate (PFS6) by iRECIST. Secondary objectives included objective response; the ratio of time to progression (TTP) on study to TTP on prior therapy (TTP2:TTP1); overall survival (OS); and safety. After minimum followup of 12 months, the PFS6 was 6% (95% CI 0-24%), with a median PFS and OS of 1.9 (95% CI 1.4-2.8) and 6.3 (2.8-10.3) months, respectively. Amongst 19 evaluable patients, two partial responses occurred in an endometrial adenocarcinoma and an undifferentiated pleomorphic sarcoma, maintained for 3.8 and 15.9 months respectively, both with TMB >20 mut/Mb. Adverse events were experienced by 19 patients (90%), with 15 patients (72%) experiencing grade 3-5 adverse events. Seven tremelimumab-related serious adverse events (grade 2-3) occurred in 5 patients. While the primary PFS6 endpoint was not met, there were two durable objective responses in rare cancers and a favourable change in disease trajectory for an additional five patients based on TTP ratio 1.3.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.T. as CEO of Omico, a non-profit organisation has received grants, consultancies or research support from Roche, Astra Zeneca, Pfizer, Eisai, Illumina, Beigene, Elevation Oncology, RedX Pharmaceuticals, Sun-Pharma, Bayer, Abbvie, George Clinical, Janssen, Merck, Kinnate, Microba, BioTessellate, Australian Unity, Foundation Medicine, Guardant, Intervenn, Amgen, Seattle Genetics and Eli Lilly. D.T. also serves on the advisory boards or committees for Canteen, UNSW SPHERE and NSW government in respect to genomics and translational medicine. The authors otherwise declare no competing financial or non-financial interests.
Figures

References
-
- Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol.35, S185–S198 (2015). - PubMed
-
- Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell144, 646–674 (2011). - PubMed
-
- Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol.22, 329–360 (2004). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

