Human papillomavirus (HPV) vaccination for the prevention of cervical cancer and other HPV-related diseases: a network meta-analysis
- PMID: 41276263
- PMCID: PMC12640750
- DOI: 10.1002/14651858.CD015364.pub2
Human papillomavirus (HPV) vaccination for the prevention of cervical cancer and other HPV-related diseases: a network meta-analysis
Abstract
Background: Cervical cancer is the fourth most common cause of cancer-related death amongst females worldwide. Persistent infection with high-risk human papillomavirus (HPV) is the key factor in cervical cancer development. HPV vaccines aim to prevent cancer by generating antibodies against HPV infection.
Objectives: To evaluate the safety and efficacy of HPV vaccines, in females and males, to prevent cervical cancer and other HPV-related diseases, in standard (pairwise) and network meta-analysis (NMA) of randomised controlled trials.
Search methods: On 10 January 2022, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and Embase. We searched Epistemonikos, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, the Health Technology Assessment database and vaccine manufacturer websites, and we checked reference lists from other relevant systematic reviews. We applied for Clinical Study Reports (CSRs) from the European Medicines Agency. An update search of electronic databases was done on 18 September 2024.
Selection criteria: We included randomised controlled trials (RCTs) regardless of language or publication status, assessing HPV vaccines pre-qualified by the World Health Organization (WHO) (Cervarix, Gardasil, Gardasil-9 and Cecolin).
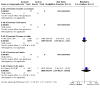
Data collection and analysis: We used methods recommended by Cochrane. We primarily used CSRs to collect data, and we included outcome data irrespective of participants' baseline HPV infection or serostatus. We assessed risk of bias using the Cochrane tool (RoB 2). All outcomes were dichotomous, and we estimated risk ratios (RR) with 95% confidence intervals (CI). We used pairwise analysis for all outcomes. Where data were available, we carried out NMA for critical outcomes for networks in females and males in three age groups, ranking the vaccines using surface under the cumulative ranking curve (SUCRA) and mean ranks. We assessed the certainty of evidence using the GRADE approach.
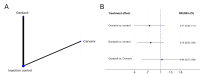
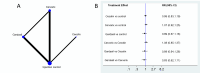
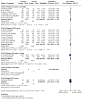
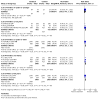
Main results: We included 60 individual studies with 157,414 participants ranging in follow-up from seven months to 11 years. Few participants were under 15. There were no studies for males under 15 years and males over 25 years. We obtained CSRs for 33 of the included studies. We assessed the risk of bias as low to 'some concerns' for the critical outcomes. Cancer and pre-cancer outcomes The studies were not of sufficient duration for cancers to develop. Four studies reported on cancer. No cancers were detected. Critical pre-cancer outcomes were reported in 15- to 25-year-old populations by 11 studies and in > 25-year-old females by three studies with up to seven years follow-up. None were reported in the under 15 years age group. In 15- to 25-year-old females, there was a reduction in CIN2+ irrespective of HPV type after six years (RR 0.70, 95% CI 0.56 to 0.88) (moderate-certainty) and a larger reduction in CIN2+ from vaccine-matched HPV types after six years (RR 0.40, 95% CI 0.30 to 0.54) (moderate-certainty). In females over 25 years old, there was little to no difference between Cervarix and Gardasil compared with control (moderate-certainty). There was no evidence on CIN2+ irrespective of HPV type from studies assessing Cecolin, or from studies assessing different dose schedules. In 15- to 25-year-old females, there was a slight reduction in vaccine-matched HPV-type high-grade vulval (VIN) or vaginal (VaIN) intraepithelial neoplasia following vaccination with Gardasil or Gardasil-9 (moderate-certainty). The NMA found a slight reduction of 1 case per 1000 following Gardasil (RR 0.21, 95% CI 0.1 to 0.45) and 0 cases per 1000 following Gardasil-9 (RR 0.16, 95% CI 0.05 to 0.51). Little to no difference was found in the NMA for Cervarix compared with control (RR 0.28, 95% CI 0.06 to 1.37), or for Cervarix, Gardasil and Gardasil-9 compared to each other. There was a reduction in high-grade anal intraepithelial neoplasia (AIN) irrespective of HPV type in the Gardasil group in one study in men who have sex with men (RR 0.75, 95% CI 0.53 to 1.07) (low-certainty). For both high-grade penile intraepithelial neoplasia (PeIN) irrespective of HPV type and vaccine-matched HPV-type high-grade PeIN, little to no difference per 1000 participants was reported in the Gardasil group in one study with 3880 participants at 36 months follow-up (RR 1.00, 95% CI 0.20 to 4.93) (low-certainty). Serious adverse events In a pairwise analysis of serious adverse events in 39 studies across all vaccine types with 97,272 participants, there was little to no difference in the HPV vaccine groups compared with the control group at up to 72 months follow-up (RR 0.99, 95% CI 0.94 to 1.04) (high-certainty). Treatment rates for HPV-related pre-invasive disease In pairwise analysis of five studies with 38,606 participants, there were 12 fewer people that needed to seek treatment per 1000 participants (95% CI 5 to 17 fewer per 1000) in the HPV vaccine groups compared with the control group rate at up to 84 months follow-up (RR 0.76, 95% CI 0.65 to 0.89) (moderate-certainty). Anogenital warts In pairwise analysis of three studies with 21,271 participants, there were 25 fewer cases of anogenital warts irrespective of HPV type per 1000 participants (95% CI 22 to 28 fewer per 1000) in the HPV vaccine groups compared with the control group rate at up to 48 months follow-up (RR 0.38, 95% CI 0.32 to 0.46) (high-certainty). In the NMA for females 15 to 25 years old, Gardasil-9 was most likely to reduce the risk of developing anogenital warts.
Authors' conclusions: The evidence in this network meta-analysis of HPV vaccines is based on extensive searches and analyses. There is evidence from randomised controlled trials that HPV vaccination reduces the risk of pre-cancerous outcomes such as CIN2+ and anogenital warts. No data were available for cervical cancer or other cancer outcomes, and no data on pre-cancer outcomes were available for vaccination under age 15 years. There were no safety concerns noted in the studies.
Trial registration: ClinicalTrials.gov NCT00956553 NCT00423046 NCT01462357 NCT00344032 NCT00426361 NCT00689741 NCT00290277 NCT00485732 NCT00316693 NCT000534638 NCT00637195 NCT00345878 NCT01627561 NCT00196924 NCT00306241 NCT00122681 NCT00578227 NCT00309166 NCT00541970 NCT00652938 NCT00481767 NCT00294047 NCT00779766 NCT00996125 NCT01277042 NCT00090285 NCT00496626 NCT01461993 NCT00365716 NCT00923702 NCT00365378 NCT00501137 NCT01717118 NCT04508309 NCT03943875 NCT01824537 NCT00520598 NCT05149248 NCT01755689 NCT04953130 NCT03180034 NCT04199689 NCT03832049 NCT02009800 NCT03998254 NCT04635423 NCT04772534 NCT05279248 NCT05415345 NCT05672927 NCT06345885 NCT05237947 NCT03105856.
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
A conflict of interest is defined as a set of conditions that pose a risk that professional judgement concerning a primary interest (such as patients' welfare or the validity of research) can be unduly influenced (consciously or unconsciously) by a secondary interest (such as financial gain) (Cochrane Library 2020).
Ingrid Arevalo‐Rodriguez: has declared that they have no conflict of interest.Hanna Bergman: has declared that they have no conflict of interest.Brian S Buckley: has declared that they have no conflict of interest.Emma J Crosbie: is an NIHR Research Professor and Honorary Consultant Gynaecological Oncologist at the University of Manchester and Manchester University NHS Foundation Trust. EJC treats patients with HPV‐related conditions, including cervical and vulval cancer and pre‐cancer. EJC reports an NIHR grant to support performing this review (academic support to perform the review from a non‐conflicted source); paid to institution. EJC is Deputy Editor in Chief for BJOG; personal payment. EJC is President of Peaches Womb Cancer Trust; unpaid. EJC is Chair of the Research Advisory Committee for The Eve Appeal; unpaid. EJC has received honoraria from GlaxoSmithKline and Astellas; personal payment. EJC has received research grants from Roche and Novosanis; paid to institution.Jen Davies‐Oliviera: has declared that they have no conflict of interest.Kerry Dwan: has declared that they have no conflict of interest.Su P Golder: has declared that they have no conflict of interest.Nicholas Henschke: has declared that they have no conflict of interest.Yoon Kong Loke: has declared that they have no conflict of interest.Jo Morrison: reports a NIHR grant to support performing this review (academic support to perform the review from a non‐conflicted source); personal payment. JM is the Co‐Chair of the British Gynaecological Cancer Society (BGCS) guidelines subgroup; unpaid position. JM has published opinions on Twitter, and co‐wrote a Cochrane editorial about a previous HPV vaccine review. JM is a consultant gynaecologist in Somerset NHS Foundation Trust. JM treats patients with HPV‐related conditions, including cervical and vulval cancer and pre‐cancer. Clinical expertise informed by the results of the studies included in the previous HPV vaccine reviews and is a member of the NHS Cervical Screening Research Advisory Committee (unpaid). JM was a Co‐ordinating Editor in Cochrane at the time of previous versions of HPV vaccine reviews. JM is a Senior Editor for Cochrane (Sexual and Reproductive Health Thematic group), although the author was not involved in the editorial process of this review.Jennifer Petkovic: has declared that they have no conflict of interest.Katrin Probyn: has declared that they have no conflict of interest.Gemma Villanueva: has declared that they have no conflict of interest.
Figures


































































































































































































































































Update of
References
References to studies included in this review
2v4v Draper 2013‐UK {published and unpublished data}EUCTR2008‐006773‐32‐GB
-
- Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A randomized, observer-blinded immunogenicity trial of Cervarix and Gardasil human papillomavirus vaccines in 12-15 year old girls. PloS One 2013;8(5):e61825.
-
- Haskins-Coulter T, Southern J, Andrews N, Miller E. Reactogenicity of Cervarix and Gardasil human papillomavirus (HPV) vaccines in a randomized single blind trial in healthy UK adolescent females. Human Vaccines and Immunotherapeutics 2017;13(6):1-9.
2v4v Einstein 2009‐USA {published and unpublished data}108933HPV‐010
-
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Human Vaccines 2009;5(10):705-19.
-
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Human Vaccines 2011;7(12):1343-58.
-
- Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study. Human Vaccines and Immunotherapeutics 2015;10(12):3455-65.
-
- Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a Phase III randomized trial. Human Vaccines and Immunotherapeutics 2014;10(12):3435-45.
-
- GlaxoSmithKline. Clinical study report: immunogenicity of GlaxoSmithKline Biological’s human papillomavirus (HPV) vaccine (580299) versus Merck’s Gardasil® in healthy females 18-45 years of age. Available from: https://www.gsk-studyregister.com/en/trial-details/?id=108933 (accessed on 03 October 2022).
2v4v Gilca 2015‐CAN {published and unpublished data}NCT01456715
-
- Gilca V, Sauvageau C, Boulianne N, De Serres G, Crajden M, Ouakki M, et al. The effect of a booster dose of quadrivalent or bivalent HPV vaccine when administered to girls previously vaccinated with two doses of quadrivalent HPV vaccine. Human Vaccines and Immunotherapeutics 2015;11(3):732-8.
2v4v Leung 2015‐INT {published and unpublished data}115411
-
- GlaxoSmith Kline. Clinical study report: immunogenicity and safety study of GlaxoSmithKline Biologicals' human papillomavirus (HPV) vaccine (GSK-580299) and Merck's Gardasil vaccine when administered according to alternative 2-dose schedules in 9-14 year old females. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=115411 (accessed on 03 October 2022).
-
- Leung TF, Liu AP, Lim FS, Thollot F, Oh HM, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 as04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9-14 years: results to month 12 from a randomized trial. Human Vaccines & Immunotherapeutics 2015;11(7):1689-702.
-
- Leung TF, Liu AP-Y, Lim FS, Thollot F, Oh HM, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and 4vHPV vaccine administered according to two- or three-dose schedules in girls aged 9-14 years: results to month 36 from a randomized trial. Vaccine 2018;36(1):98-106.
2v4v Nelson 2013‐HKG {published and unpublished data}
-
- Nelson EA, Lam HS, Choi KC, Ho WC, Fung LW, Cheng FW, et al. A pilot randomized study to assess immunogenicity, reactogenicity, safety and tolerability of two human papillomavirus vaccines administered intramuscularly and intradermally to females aged 18-26 years. Vaccine 2013;31(34):3452-60.
2v4v Sangar 2015‐IND {published and unpublished data}
-
- Sangar VC, Ghongane BB, Gupte R, Kesarkar R, Kalyan K, Chowdhary A. Comparison of post-licensure safety surveillance of bivalent and quadrivalent human papillomavirus vaccines in healthy Mumbai women. International Journal of Pharmacy and Pharmaceutical Sciences 2015;7(3):437-42.
2v9v DoRIS 2022‐TZN {published and unpublished data}NCT02834637
-
- Watson-Jones D, Changalucha J, Whitworth H, Pinto L, Mutani P, Indangasi J, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. Lancet Global Health 2022;10(10):e1473-e1484.
2v9v Gilca 2018‐CAN {published and unpublished data}NCT02567955
-
- Gilca V, Sauvageau C, Panicker G, De Serres G, Ouakki M, Unger ER. Immunogenicity and safety of a mixed vaccination schedule with one dose of nonavalent and one dose of bivalent HPV vaccine versus two doses of nonavalent vaccine - a randomized clinical trial. Vaccine 2018;36(46):7017-24.
2v9v KENSHE 2021‐KEN {published and unpublished data}NCT03675256
-
- Barnabas RV, Brown ER, Onono MA, Bukusi EA, Njoroge B, Winer RL, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evidence 2022;1(5):EVIDoa2100056.
2v Bhatla 2010‐IND {published and unpublished data}104479HPV‐031
-
- Bhatla N, Suri V, Basu P, Shastri S, Datta SK, Bi D, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine in healthy Indian women. Journal of Obstetrics and Gynaecology Research 2010;36(1):123-32.
-
- GlaxoSmithKline. Clinical study report: evaluation of the immune and safety response of GlaxoSmithKline (GSK) Biologicals' HPV vaccine in healthy Indian women. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=104479 (accessed on 04 October 2022).
2v Carozzi 2016‐ITA {published and unpublished data}NCT02296255
-
- Carozzi FM, Ocello C, Burroni E, Faust H, Zappa M, Paci E, et al. Effectiveness of HPV vaccination in women reaching screening age in Italy. Journal of Clinical Virology 2016;84:74-81.
-
- Levi M, Bonanni P, Burroni E, Bechini A, Boccalini S, Sani C, et al. Evaluation of bivalent human papillomavirus (HPV) vaccine safety and tolerability in a sample of 25 year old Tuscan women. Human Vaccines & Immunotherapeutics 2013;9(7):1407-12.
2v CVT 2011‐CRI {published and unpublished data}NCT00128661
-
- Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. Journal of the National Cancer Institute 2015;108(1):djv302.
-
- Harari A, Chen Z, Rodriguez AC, Hildesheim A, Porras C, Herrero R, et al. Cross-protection of the bivalent human papillomavirus (HPV) vaccine against variants of genetically related high-risk HPV infections. Journal of Infectious Diseases 2016;213(6):939-47.
-
- Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008;26(37):4795-808.
-
- Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PloS One 2013;8(7):e68329.
-
- Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discovery 2011;1(5):408-19.
2v Garcia‐Sicilia 2010‐EU {published and unpublished data}108464HPV‐042
-
- Garcia-Sicilia J, Schwarz TF, Carmona A, Peters K, Malkin JE, Tran PM, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine coadministered with combined diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine to girls and young women. Journal of Adolescent Health 2010;46(2):142-51.
-
- GlaxoSmithKline. Clinical study report: evaluation of safety and immunogenicity of co-administering human papillomavirus (HPV) vaccine with other vaccines in healthy female subjects. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=108464 (accessed on 04 October 2022).
2v Harper 2004‐BRA/NA {published and unpublished data}580299/001HPV‐001
-
- De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010;28(38):6247-55.
-
- GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009;374(9706):1975-85.
-
- GlaxoSmithKline. Clinical study report: efficacy study of HPV-16/18 vaccine (GSK 580299) to prevent HPV-16 and/or -18 cervical infection in young healthy women. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=580299/001 (accessed on 04 October 2022).
-
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004;364(9447):1757-65.
-
- Harper DM, Franco EL, Wheeler CM, Moscicki A-B, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 45 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006;367(9518):1247-55.
2v Khatun 2012‐BGD {published data only}
-
- Choudhury S, Hussain S, Ferdous J, Hossain F, Begum SR, Jahan M, et al. Safety and immunogenicity profile of human papilloma virus 16/18-AS04 adjuvant cervical cancer vaccine in healthy adolescent girls of Bangladesh. In: European Journal of Cancer. Vol. 2011 European Multidisciplinary Cancer Congress. Stockholm, Sweden. 2011:S541.
-
- Hossain F. Safety and immunogenicity profile of human papillomavirus vaccine in adolescent girls of Bangladesh. International Journal of Gynaecology and Obstetrics 2012;119:S371-S372.
-
- Khatun S, Akram Hussain SM, Chowdhury S, Ferdous J, Hossain F, Begum SR, et al. Safety and immunogenicity profile of human papillomavirus-16/18 AS04 adjuvant cervical cancer vaccine: a randomized controlled trial in healthy adolescent girls of Bangladesh. Japanese Journal of Clinical Oncology 2012;42(1):36-41.
2v Kim 2010‐KOR {published and unpublished data}104951HPV‐033
-
- GlaxoSmithKline. Clinical study report: evaluate the immunogenicity & safety of GSK Biologicals' HPV vaccine in female subjects aged 10-14 years. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=104951 (accessed on 04 October 2022).
-
- Kim YJ, Kim KT, Kim JH, Cha SD, Kim JW, Bae DS, et al. Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in Korean girls aged 10-14 years. Journal of Korean Medical Science 2010;25(8):1197-204.
2v Kim 2011‐KOR {published and unpublished data}107291HPV‐038
-
- GlaxoSmithKline. Clinical study report: a study to evaluate the immune response and safety of GSK Biologicals' HPV-16/18 L1 VLP AS04 vaccine/Cervarix TM vaccine in healthy females aged 15-25 years. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=107291 (accessed on 04 October 2022).
-
- Kim SC, Song YS, Kim Y-T, Kim YT, Ryu K-S, Gunapalaiah B, et al. Human papillomavirus 16/18 AS04-adjuvanted cervical cancer vaccine: immunogenicity and safety in 15-25 years old healthy Korean women. Journal of Gynecologic Oncology 2011;22(2):67-75.
2v Konno 2010‐JPN {published and unpublished data}104798HPV‐032
-
- GlaxoSmithKline. Clinical study report: human papillomavirus (HPV) vaccine (Cervarix TM) efficacy, immunogenicity & safety trial in adult Japanese women with GSK Biologicals HPV-16/18 vaccine. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=104798 (accessed on 04 October 2022).
-
- Konno R, Dobbelaere KO, Godeaux OO, Tamura S, Yoshikawa H. Immunogenicity, reactogenicity, and safety of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women: interim analysis of a phase II, double-blind, randomized controlled trial at month 7. International Journal of Gynecological Cancer 2009;19(5):905-11. [CLINICALTRIALS.GOV: NCT00316693/ClinicalTrials.gov]
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: interim analysis of a phase 2 double-blind, randomized, controlled trial. International Journal of Gynecological Cancer 2010;20(3):404-10.
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus type 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years. International Journal of Gynecological Cancer 2010;20(5):847-55.
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Prevalence and type distribution of human papillomavirus in healthy Japanese women aged 20 to 25 years old enrolled in a clinical study. Cancer Science 2011;102(4):877-82.
2v Lehtinen 2018‐FIN {published and unpublished data}106636HPV‐040
-
- Bi D, Apter D, Eriksson T, Hokkanen M, Zima J, Damaso S, et al. Safety of the AS04-adjuvanted human papillomavirus (HPV)-16/18 vaccine in adolescents aged 12-15 years: end-of-study results from a community-randomized study up to 6.5 years. Human Vaccines & Immunotherapeutics 2019;16(6):1-12.
-
- GlaxoSmithKline. Clinical study report: effectiveness, safety and immunogenicity of GSK biologicals' HPV vaccine GSK580299 (cervarix) administered in healthy adolescents. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=106636 (accessed on 03 October 2022).
-
- Gray P, Luostarinen T, Vanska S, Eriksson T, Lagheden C, Man I, et al. Occurrence of human papillomavirus (HPV) type replacement by sexual risk-taking behaviour group: post-hoc analysis of a community randomized clinical trial up to 9 years after vaccination (IV). International Journal of Cancer 2019;145(3):785-96.
-
- Gray P, Palmroth J, Luostarinen T, Apter D, Dubin G, Garnett G, et al. Evaluation of HPV type-replacement in unvaccinated and vaccinated adolescent females - post-hoc analysis of a community-randomized clinical trial (II). International Journal of Cancer 2018;142(12):2491-500.
-
- Kalliala I, Eriksson T, Aro K, Hokkanen M, Lehtinen M, Gissler M, et al. Preterm birth rate after bivalent HPV vaccination: registry-based follow-up of a randomized clinical trial. Preventive Medicine 2021;146:106473.
2v Leroux‐Roels 2011‐BEL {published and unpublished data}111567HPV‐026‐PRI
-
- GlaxoSmithKline. Clinical study report: immunogenicity and safety of a commercially available vaccine co-administered with GSK HPV vaccine (580299). Available at: https://www.gsk-studyregister.com/en/trial-details/?id=111567 (accessed on 04 October 2022).
-
- Leroux-Roels G, Haelterman E, Maes C, Levy J, De Boever F, Licini L, et al. Randomized trial of the immunogenicity and safety of the Hepatitis B vaccine given in an accelerated schedule coadministered with the human papillomavirus type 16/18 AS04-adjuvanted cervical cancer vaccine. Clinical and Vaccine Immunology 2011;18(9):1510-8.
2v Lim 2014‐MYS {published and unpublished data}105926HPV‐036
-
- GlaxoSmithKline. Clinical study report: study to evaluate the immune response and safety of GSK Biologicals' HPV vaccine in healthy women aged 18-35 years. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=105926 (accessed on 04 October 2022).
-
- Lim BK, Ng KY, Omar J, Omar SZ, Gunapalaiah B, Teoh YL, et al. Immunogenicity and safety of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine in Malaysian women aged 18-35 years: a randomized controlled trial. Medical Journal of Malaysia 2014;69(1):2-8.
2v Lin 2018‐LA {published and unpublished data}115887HPV‐073
-
- GlaxoSmithKline. Clinical study report: safety and immunogenicity of GSK Biologicals’ HPV-16/18 L1 VLP AS04 vaccine (GSK-580299) in healthy female children 4-6 years old. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=115887 (accessed on 04 October 2022).
-
- Lin L, Macias Parra M, Sierra VY, Salas Cespedes A, Granados MA, Luque A, et al. Long-term immunogenicity and safety of the AS04-adjuvanted human papillomavirus-16/18 vaccine in four- to six-year-old girls: three-year follow-up of a randomized phase III trial. Pediatric Infectious Disease Journal 2019;38(10):1061-7.
-
- Lin L, Parra MM, Sierra VY, Cespedes AS, Granados MA, Luque A, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in 4-6-year-old girls: results to month 12 from a randomized trial. Pediatric Infectious Disease Journal 2018;37(4):e93-e102.
-
- Lin L, Parra MM, Sierra VY, Cespedes AS, Granados MA, Luque A, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in 4-6-year-old girls: results to month 12 from a randomized trial. Pediatric Infectious Disease Journal 2018;37(4):e93-e102.
2v Medina 2010‐INT {published and unpublished data}580299/013HPV‐013
-
- GlaxoSmithKline. Clinical study report: human papillomavirus vaccine safety and immunogenicity trial in young adolescent women with GSK Bio HPV-16/18. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=580299/013 (accessed on 04 October 2022).
-
- Medina DM, Valencia A, Velasquez A, Huang LM, Prymula R, Garcia-Sicilia J, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: a randomized, controlled trial in adolescent girls. Journal of Adolescent Health 2010;46(5):414-21.
-
- Schwarz TF, Huang L-M, Medina DM, Valencia A, Lin T-Y, Behre U, et al. Four-year follow-up of the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine when administered to adolescent girls aged 10-14 years. Journal of Adolescent Health 2012;50(2):187-94.
-
- Schwarz TF, Huang LM, Lin TY, Wittermann C, Panzer F, Valencia A, et al. Long-term immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in 10- to 14-year-old girls: open 6-year follow-up of an initial observer-blinded, randomized trial. Pediatric Infectious Disease Journal 2014;33(12):1255-61.
2v Ngan 2010‐HKG {published and unpublished data}106001HPV‐035
-
- GlaxoSmithKline. Clinical study report: a study to evaluate the immunogenicity and safety of GSK Biologicals' HPV vaccine in healthy women aged 18-35 years. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=106001 (accessed on 04 October 2022).
-
- Ngan HY, Cheung AN, Tam KF, Chan KK, Tang HW, Bi D, et al. Human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine: immunogenicity and safety in healthy Chinese women from Hong Kong. Xianggang Yi Xue za Zhi [Hong Kong Medical Journal] 2010;16(3):171-9.
2v PATRICIA 2012‐INT {published and unpublished data}580299HPV‐008
-
- Apter D, Wheeler CM, Paavonen J, Castellsague X, Garland SM, Skinner SR, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clinical and Vaccine Immunology 2015;22(4):361-73.
-
- Budenholzer B. HPV-16/18 AS04-adjuvanted vaccine prevented cervical intraepithelial neoplasia > grade 3 in young women. Annals of Internal Medicine 2012;157(2):JC2‐7.
-
- Frederick PJ, Huh WK, PATRICIA Study Group. Evaluation of the interim analysis from the PATRICIA study group: efficacy of a vaccine against HPV 16 and 18. Expert Review of Anticancer Therapy 2008;8(5):701-5. Erratum appears in Expert Review of Anticancer Therapy 2008 Jun;8(6):1014.
-
- Garland S, Paavonen J, Teixeira J, Hedrick J, Struyf F, Dubin G. Cross-protective efficacy of Cervarix against HPV-45 in a double blind randomized controlled Phase III efficacy trial. In: International Journal of Gynaecology and Obstetrics. Vol. Conference: 19th FIGO World Congress of Gynecology and Obstetrics. Cape Town, South Africa. 2009:S188.
-
- Garland SM. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against vulvar/vaginal intraepithelial neoplasia. Journal of Lower Genital Tract Disease 2013;17(6 Suppl 2):S110.
2v Pedersen 2012‐NA/EU {published and unpublished data}110886HPV‐029‐PRI
-
- GlaxoSmithKline. Clinical study report: evaluation of safety and immunogenicity of co-administering human papillomavirus vaccine with another vaccine in healthy female subjects. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=110886 (accessed on 04 October 2022).
-
- Pedersen C, Breindahl M, Aggarwal N, Berglund J, Oroszlan G, Silfverdal SA, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. Journal of Adolescent Health 2012;50(1):38-46.
2v Petaja 2009‐FIN {published and unpublished data}580299/011HPV‐011
-
- GlaxoSmithKline. Clinical study report: evaluation of the immunogenicity and safety of GlaxoSmithKline Biologicals' HPV vaccine in young males. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=580299/011 (accessed on 03 October 2022).
-
- Petaja T, Keranen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10-18 years. Journal of Adolescent Health 2009;44(1):33-40.
2v Romanowski 2011‐CAN/GER {published and unpublished data}110659
-
- GlaxoSmithKline. Clinical study report: partially blind study to evaluate immunogenicity & safety of GSK Bio's HPV vaccine 580299 in healthy women aged 9-25 yrs. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=110659 (accessed on 04 October 2022).
-
- Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: five-year clinical data and modeling predictions from a randomized study. Human Vaccines and Immunotherapeutics 2016;12(1):20-9.
-
- Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Human Vaccines and Immunotherapeutics 2014;10(5):1155-65.
-
- Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Human Vaccines 2011;7(12):1374-86.
-
- Schwarz T, Romanowski B, Peters K, Dionne M, Schulze K, Ramjattan B, et al. Immune response to the hpv-16/18 as04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 3 years after vaccination. International Journal of Gynaecology and Obstetrics 2012;119:S476.
2v Schmeink 2011‐NLD/SWE {published and unpublished data}111507HPV‐030
-
- GlaxoSmithKline. Clinical study report: evaluation of immunogenicity and safety of human papillomavirus (HPV) vaccine co-administered with another vaccine in healthy female subjects. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=111507 (accessed on 04 October 2022).
-
- Schmeink CE, Bekkers RL, Josefsson A, Richardus JH, Berndtsson Blom K, David MP, et al. Co-administration of human papillomavirus-16/18 AS04-adjuvanted vaccine with hepatitis B vaccine: randomized study in healthy girls. Vaccine 2011;29(49):9276-83.
2v Sow 2013‐SEN/TZN {published and unpublished data}106069HPV‐021
-
- GlaxoSmithKline. Clinical study report: immunogenicity and safety of GlaxoSmithKline (GSK) Biologicals’ human papillomavirus (HPV) vaccine (GSK580299) in healthy female subjects 10-25 years of age. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=106069 (accessed on 04 October 2022).
-
- Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women. Journal of Infectious Diseases 2013;207(11):1753-63.
2v VIVIANE 2014‐INT {published and unpublished data}104820HPV‐015
-
- GlaxoSmithKline. Clinical study report: study to evaluate the efficacy of the human papillomavirus vaccine in healthy adult women of 26 years of age and older. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=104820 (accessed on 04 October 2022).
-
- Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmeron J, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet 2014;384(9961):2213-27.
-
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infectious Diseases 2016;16(10):1154–68.
2v Zhu 2014‐CHNa {published and unpublished data}107638HPV‐039
-
- GlaxoSmithKline. Clinical study report: efficacy, immunogenicity and safety of GSK Biologicals’ HPV GSK 580299 vaccine in healthy Chinese female subjects. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=107638 (accessed on 04 October 2022).
-
- Hu S, Xu X, Zhu F, Hong Y, Hu Y, Zhang X, et al. Efficacy of the AS04-adjuvanted HPV-16/18 vaccine in young Chinese women with oncogenic HPV infection at baseline: post-hoc analysis of a randomized controlled trial. Human Vaccines and Immunotherapeutics 2020;17(4):955-64.
-
- Zhu F, Zhao F, Chen W, Hu S, Hu Y, Hong Y, et al. Efficacy, safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in chinese women: 4-year follow-up results from a randomized controlled trial. International Journal of Gynecological Cancer 2014;24(9 Suppl 4):1584-5.
-
- Zhu F-C, Hu S-Y, Hong Y, Hu Y-M, Zhang X, Zhang Y-J, et al. Efficacy, immunogenicity and safety of the AS04-HPV-16/18 vaccine in Chinese women aged 18-25A years: end-of-study results from a phase II/III, randomised, controlled trial. Cancer Medicine 2019;8(14):6195-211.
-
- Zhu F-C, Hu S-Y, Hong Y, Hu Y-M, Zhang X, Zhang Y-J, et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18-25 years: event-triggered analysis of a randomized controlled trial. Cancer Medicine 2017;6(1):12-25.
2v Zhu 2014‐CHNb {published and unpublished data}112022HPV‐058
-
- GlaxoSmithKline. Clinical study report: immunogenicity and safety study of GSK Biologicals’ human papillomavirus 580299 vaccine in healthy female subjects. Available at: https://www.gsk-studyregister.com/en/trial-details/?id=112022 (accessed on 04 October 2022).
-
- NCT00996125. Immunogenicity and safety study of GSK biologicals' human papillomavirus 580299 vaccine in healthy female subjects. https://clinicaltrials.gov/show/NCT00996125 2009.
-
- Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial. International Journal of Cancer 2014;135(11):2612-22.
2v Zhu 2014‐CHNc {published and unpublished data}114590HPV‐069‐PRI
-
- GlaxoSmithKline. Clinical study report: study to assess immune responses and safety of the GSK-580299 vaccine in healthy women (26 to 45 years). Available at: https://www.gsk-studyregister.com/en/trial-details/?id=114590 (accessed on 04 October 2022).
-
- NCT01277042. Study to assess immune responses and safety of the GSK-580299 vaccine in healthy women (26 to 45 years). https://clinicaltrials.gov/show/NCT01277042 2011.
-
- Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial. International Journal of Cancer 2014;135(11):2612-22.
4v9v Garland 2015‐INT {published and unpublished data}V503‐006NCT01047345
-
- Garland SM, Cheung TH, McNeill S, Petersen LK, Romaguera J, Vazquez-Narvaez J, et al. Safety and immunogenicity of a 9-valent HPV vaccine in females 12-26 years of age who previously received the quadrivalent HPV vaccine. Vaccine 2015;33(48):6855-64.
-
- Merck. Clinical study report: a phase III randomized, international, placebo-controlled, double-blind clinical trial to study the tolerability and immunogenicity of V503, a multivalent human papillomavirus (HPV) L1 virus-like particle (VLP) vaccine, given to females 12-26 years of age who have previously received GARDASIL (Protocol 006). European Medicines Agency.
4v9v Joura 2015‐INT {published and unpublished data}NCT00543543
-
- Guevara A, Cabello R, Woelber L, Moreira ED, Joura E, Reich O. Antibody persistence and evidence of immune memory at 5 years following administration of the 9-valent HPV vaccine. Vaccine 2017;35:5050‐7.
-
- Huh WK, Joura EA, Giuliano AR, Iversen O-E, Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet 2017;390(10108):2143-59.
-
- Joura E, Garland S, Giuliano A, Bautista O, Chen J, Moeller E, et al. End of study efficacy for vulvovaginal disease of a novel 9-valent HPV L1 virus-like particle vaccine in 16-26 year old women. Journal of Lower Genital Tract Disease 2015;19(3 Suppl 1):S10.
-
- Joura E, Giuliano A, Iversen OE, Bautista O, Chen J, Moeller E, et al. End of study efficacy and immunogenicity of a novel 9-valent HPV L1 virus-like particle vaccine in 16-26 year old women. EUROGIN 2015 Abstracts: HPV Infection and Related Cancers 2015;OC6:174.
-
- Joura E, Vuocolo S. Efficacy of a novel 9-valent HPV vaccine against high-grade lesions and cancer in 16-to 26-year-old women. International Journal of Gynecological Cancer 2014;24(9 Suppl 4):32.
4v9v Van Damme 2016‐EU {published and unpublished data}NCT02114385
-
- Van Damme P, Meijer C, Kieninger D, Schuyleman A, Thomas S, Luxembourg A, et al. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine 2016;34(35):4205-12.
4v9v Vesikari 2015‐EU {published and unpublished data}NCT01304498
-
- Vesikari T, Brodszki N, Van Damme P, Diez-Domingo J, Icardi G, Petersen LK, et al. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus Gardasil in 9-15 year-old girls. Pediatric Infectious Disease Journal 2015;34(9):992-8.
4v Chang 2020‐USA {published and unpublished data}NCT02199691
-
- Chang L-J, Hedrick J, Christensen S, Pan J, Jordanov E, Dhingra MS. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine 2020;38(19):3560-9.
4v Dobson 2013‐CAN {published and unpublished data}NCT00501137
-
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309(17):1793-802.
-
- Merck. Clinical study report: a controlled trial to assess the immunogenicity of a 2-dose schedule of human papillomavirus vaccine. Health Canada.
4v EVRI 2016‐ZAF {published and unpublished data}NCT01489527
-
- Giuliano AR, Botha MH, Zeier M, Abrahamsen ME, Glashoff RH, Van der Laan LE, et al. High HIV, HPV, and STI prevalence among young western cape, south african women: EVRI HIV prevention preparedness trial. Journal of Acquired Immune Deficiency Syndromes (1999) 2015;68(2):227-35.
-
- Menezes LJ, Pokharel U, Sudenga SL, Botha MH, Zeier M, Abrahamsen ME, et al. Patterns of prevalent HPV and STI co-infections and associated factors among HIV-negative young Western Cape, South African women: the EVRI trial. Sexually Transmitted Infections 2018;94(1):55-61.
-
- Sudenga SL, Torres BN, Botha MH, Zeier M, Abrahamsen ME, Glashoff RH, et al. Cervical HPV natural history among young western cape, south african women: the randomized control EVRI trial. Journal of Infection 2016;72(1):60-9.
-
- Sudenga SL, Torres BN, Botha MH, Zeier M, Abrahamsen ME, Glashoff RH, et al. HPV serostatus pre- and post-vaccination in a randomized phase II preparedness trial among young Western Cape, South African women: the EVRI trial. Papillomavirus Research 2017;3:50-6.
4v Foresta 2015‐ITA {published data only}
-
- Foresta C, Garolla A, Parisi S, Ghezzi M, Bertoldo A, Di Nisio A, et al. HPV prophylactic vaccination in males improves the clearance of semen infection. EBioMedicine 2015;2(10):1487-93.
4v FUTURE 2007‐INT {published and unpublished data}NCT00092521
-
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New England Journal of Medicine 2007;356(19):1928-43.
-
- Merck. Clinical study report: a study to evaluate the efficacy of quadrivalent HPV (types 6, 11, 16, and 18) L1 virus-like particle (VLP) vaccine in reducing the incidence of HPV 6-, 11-, 16-, and 18-related CIN, AIS, and cervical cancer, and HPV 6-, 11-, 16-, and 18-related external genital warts, VIN, VaIN, vulvar cancer, and vaginal cancer in 16- to 23-year-old women – the FUTURE I study (females united to unilaterally reduce endo/ectocervical disease). European Medicines Agency.
-
- Paavonen J. Long-term efficacy of human papillomavirus vaccination against cervical cancer. Sexually Transmitted Infections 2013;89(Suppl 1):A53.
-
- Wheeler CM, Bautista OM, Tomassini JE, Nelson M, Sattler CA, Barr E, Protocol 11 study Investigators. Safety and immunogenicity of co-administered quadrivalent human papillomavirus (HPV)-6/11/16/18 L1 virus-like particle (VLP) and hepatitis B (HBV) vaccines. Vaccine 2008;26(5):686-96.
4v FUTURE I/II 2010‐INT {published data only}
-
- FUTURE I/II Study Group. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ (Clinical Research Ed.) 2010;341:c3493.
-
- Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. Journal of the National Cancer Institute 2010;102(5):325-39.
4v FUTURE II 2007‐INT {published and unpublished data}NCT00092534
-
- Enerly E, Berger S, Kjaer SK, Sundstrom K, Campbell S, Tryggvadottir L, et al. Use of real-world data for HPV vaccine trial follow-up in the Nordic region. Contemporary Clinical Trials 2020;92:105996.
-
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. New England Journal of Medicine 2007;356(19):1915-27.
-
- Kjaer SK, Nygard M, Dillner J, Marshall JB, Radley D, Li M, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 nordic countries. Clinical Infectious Diseases 2018;66(3):339-45.
-
- Kjaer SK, Nygard M, Sundstrom K, Dillner J, Tryggvadottir L, Munk C, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine 2020;23:100401.
-
- Paavonen J, Rana M, Apter D, Luostarinen T, Pukkala E, Lehtinen M. Long-term efficacy of human papillomavirus vaccination against cin3 and invasive carcinoma: registry based follow-up of a phase III trial (future II). Sexually Transmitted Infections 2011;87(Suppl 1):A71-A72.
4v FUTURE III 2009‐INT {published and unpublished data}NCT00090220
-
- Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. British Journal of Cancer 2011;105(1):28-37.
-
- Ferris DG, Brown DR, Giuliano AR, Myers E, Joura EA, Garland SM, et al. Prevalence, incidence, and natural history of HPV infection in adult women ages 24 to 45 participating in a vaccine trial. Papillomavirus Research 2020;10:100202.
-
- Ferris DG. The effect of quadrivalent HPV (types 6/11/16/18) vaccination on Papanicolaou smears and cervical procedures in women aged 24 to 45. Journal of Lower Genital Tract Disease 2010;14(3):245.
-
- Luna J, Plata M, Gonzalez M, Correa A, Maldonado I, Nossa C, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil in adult women. PloS One 2013;8(12):e83431.
-
- Makhija S. Efficacy of quadrivalent HPV 6/11/16/18 vaccine against persistent infection or disease in subjects with prior vaccine HPV type infection. Gynecologic Oncology 2010;116(3 Suppl 1):S60-S61.
4v Giuliano 2011‐INT {published and unpublished data}V501‐020
-
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. New England Journal of Medicine 2011;364(5):401-11.
-
- Goldstone S, Giuliano A, Palefsky J, Luxembourg A. Long-term effectiveness and immunogenicity of quadrivalent HPV vaccine in young men: 10-year end-of study analysis. Journal of Clinical Oncology 2018;36(15 Suppl):1553.
-
- Goldstone S. Efficacy of the quadrivalent HPV vaccine to prevent anal intraepithelial neoplasia among young men who have sex with men. Sexually Transmitted Infections 2011;87(Suppl 1):A352.
-
- Goldstone SE, Giuliano AR, Palefsky JM, Lazcano-Ponce E, Penny ME, Cabello RE, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infectious Diseases 2022;22(3):413-25.
-
- Goldstone SE, Jessen H, Palefsky JM, Giuliano AR, Moreira ED, Vardas E, et al. Quadrivalent HPV vaccine efficacy against disease related to vaccine and non-vaccine HPV types in males. Vaccine 2013;31(37):3849-55.
4v Kang 2008‐KOR {published and unpublished data}NCT00157950
-
- Kang S, Kim KH, Kim YT, Kim YT, Kim JH, Song YS, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, placebo-controlled trial in 176 Korean subjects. International Journal of Gynecological Cancer 2008;18(5):1013-9.
4v Li 2012‐CHN {published and unpublished data}
-
- Li R, Li Y, Radley D, Liu Y, Huang T, Sings HL, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double-blind, placebo-controlled trial in Chinese males and females. Vaccine 2012;30(28):4284-91.
4v Mikamo 2019‐JPN {published and unpublished data}NCT01862874
-
- Mikamo H, Yamagishi Y, Murata S, Yokokawa R, Han SR, Wakana A, et al. Efficacy, safety, and immunogenicity of a quadrivalent HPV vaccine in Japanese men: a randomized, Phase 3, placebo-controlled study. Vaccine 2019;37(12):1651-8.
4v Mugo 2015‐AF {published and unpublished data}NCT01245764
-
- Mugo N, Ansah NA, Marino D, Saah A, Garner EI. Evaluation of safety and immunogenicity of a quadrivalent human papillomavirus vaccine in healthy females between 9 and 26 years of age in Sub-Saharan Africa. Human Vaccines & Immunotherapeutics 2015;11(6):1323-30.
4v NCT00411749 2006‐JPN {unpublished data only}NCT00411749
-
- NCT00411749. V501 immunogenicity study in females age 9 to 17 years (V501-028). https://clinicaltrials.gov/ct2/show/study/NCT00411749 2006.
4v Reisinger 2007‐INT {published and unpublished data}NCT00092547
-
- Merck. Clinical study report: a long term immunogenicity, safety, and effectiveness study of GARDASIL™ (human papillomavirus [types 6,11,16,18] recombinant vaccine) among adolescents who received GARDASIL™ at 9-18 years of age. European Medicines Agency.
-
- Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatric Infectious Disease Journal 2007;26(3):201-9.
4v Senders 2016‐USA {published data only}
-
- NCT01461993. A clinical trial to study the safety, tolerance and immunogenic response to Gardasil and bivalent rlp2086 vaccine when given at the same time to children between the ages of 11 and 17. https://clinicaltrials.gov/show/NCT01461993 2011.
-
- Senders S, Bhuyan P, Jiang Q, Absalon J, Eiden JJ, Jones TR, et al. Immunogenicity, tolerability and safety in adolescents of bivalent rlp2086, a meningococcal serogroup B vaccine, coadministered with quadrivalent human papilloma virus vaccine. Pediatric Infectious Disease Journal 2016;35(5):548-54.
4v Villa/FUTURE I/II 2009‐INT {published data only}
-
- Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 2007;369(9574):1693-702.
-
- Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(10):868-78.
4v Villa 2005‐INT {published and unpublished data}
-
- Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006;24(27-28):5571-83.
-
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet. Oncology 2005;6(5):271-8.
-
- Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. British Journal of Cancer 2006;95(11):1459-66.
4v Wei 2019‐CHN {published and unpublished data}NCT00834106
-
- Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, et al. Safety of a quadrivalent human papillomavirus vaccine in a Phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine 2019;37(6):889-97.
-
- Chen W, Zhao Y, Xie X, Liu J, Liao X, Shou Q, et al. Safety and efficacy of a quadrivalent HPV (QHPV) vaccine in Chinese women: results of base study with 30-month follow-up. International Journal of Gynecological Cancer 2017;27 (Suppl 4):136.
-
- Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine 2019;37(27):3617-24.
-
- Zhao C, Wei L, Li J, Zhao Y, Li M. Long-term efficacy of quadrivalent human papillomavirus vaccine in Chinese women during 10 years of follow-up. Journal of Lower Genital Tract Disease 2020;24(Suppl 1):S21.
4v Yoshikawa/NCT00411749 2013‐JPN {published data only}
-
- Murata S, Shirakawa M, Sugawara Y, Shuto M, Sawata M, Tanaka Y. Post-hoc analysis of injection-site reactions following vaccination with quadrivalent human papillomavirus vaccine in Japanese female clinical trial participants. Papillomavirus Research 2020;10:100205.
4v Yoshikawa 2013‐JPN {published and unpublished data}NCT00378560
-
- Yoshikawa H, Ebihara K, Tanaka Y, Noda K. Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (GARDASIL) in Japanese women aged 18-26 years. Cancer Science 2013;104(4):465-72.
9v Iversen 2016‐INT {published and unpublished data}NCT01984697
-
- Bornstein J, Roux S, Petersen LK, Huang L-M, Dobson SR, Pitisuttithum P, et al. Three-year follow-up of 2-dose versus 3-dose HPV vaccine. Pediatrics 2021;147(1):e20194035.
-
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA 2016;316(22):2411-21.
-
- Merck. Clinical study report: a phase III clinical trial to study the tolerability and immunogenicity of a 2-dose regimen of V503, a multivalent human papillomavirus (HPV) L1 virus-like particle (VLP) vaccine, administered in preadolescents and adolescents (9 to 14 year olds) with a comparison to young women (16 to 26 year olds). Health Canada.
Cecolin 2v Hu 2020‐CHN {published and unpublished data}NCT02562508
-
- Hu Y-M, Guo M, Li C-G, Chu K, He W-G, Zhang J, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Science China Life Sciences 2020;63(4):582-91.
Cecolin 2v Qiao 2020‐CHN {published and unpublished data}NCT01735006
-
- Qiao Y-L, Wu T, Li R-C, Hu Y-M, Wei L-H, Li C-G, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. Journal of the National Cancer Institute 2020;112(2):145-53.
-
- Su Y-Y, Lin B-Z, Zhao H, Li J, Lin Z-J, Qiao Y-L, et al. Lot-to-lot consistency study of an Escherichia coli-produced bivalent human papillomavirus vaccine in adult women: a randomized trial. Human Vaccines and Immunotherapeutics 2019;16(7):1636-44.
Cecolin 2v Wu 2015‐CHN {published and unpublished data}NCT01356823
-
- Wu T, Hu Y-M, Li J, Chu K, Huang S-J, Zhao H, et al. Immunogenicity and safety of an E. coli-produced bivalent human papillomavirus (type 16 and 18) vaccine: a randomized controlled phase 2 clinical trial. Vaccine 2015;33(32):3940-6.
References to studies excluded from this review
Basu 2021 {published data only}
-
- Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncology 2021;22(11):1518-29.
Brown 2012 {published data only}
-
- Brown B, Blas M, Cabral A, Carcamo C, Gravitt P, Halsey N. Randomized trial of HPV4 vaccine assessing the response to HPV4 vaccine in two schedules among Peruvian female sex workers. Vaccine 2012;30(13):2309-14.
Chen 2020 {published data only}
-
- Chen Q, Zhao H, Yao XM, Lin ZJ, Li J, Lin BZ, et al. Comparing immunogenicity of the Escherichia coli-produced bivalent human papillomavirus vaccine in females of different ages. Vaccine 2020;38(39):6096-102.
Donken 2019 {published data only}
-
- Donken R, Dobson SR, Marty KD, Cook D, Sauvageau C, Gilca V, et al. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clinical Infectious Diseases 2019;71(4):1022-9.
Emeny 2002 {published data only}
-
- Emeny RT, Wheeler CM, Jansen KU, Hunt WC, Fu TM, Smith JF, et al. Priming of human papillomavirus type 11-specific humoral and cellular immune responses in college-aged women with a virus-like particle vaccine. Journal of Virology 2002;76(15):7832-42.
Esposito 2011 {published data only}
-
- Esposito S, Birlutiu V, Jarcuska P, Perino A, Man SC, Vladareanu R, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: results from a randomized study. Pediatric Infectious Disease Journal 2011;30(3):e49-55.
EUCTR2012‐004007‐13 {published data only}
-
- EUCTR2012-004007-13. A randomized, placebo-controlled, phase IIIb HPV vaccination trial with Gardasil® in patients with recurrent condylomata acuminata. https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-004007-13/DE (accessed on 11 December 2024) 2013.
Feder 2019 {published data only}
-
- Feder MA, Kulasingam SL, Kiviat NB, Mao C, Nelson EJ, Winer RL, et al. Correlates of human papillomavirus vaccination and association with HPV-16 and HPV-18 DNA detection in young women. Journal of Women's Health 2019;28(10):1428-35.
Ferris 2014 {published data only}
-
- Ferris D, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Reisinger KS, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014;134(3):e657-65.
Ferris 2017 {published data only}
-
- Ferris DG, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Mehlsen J, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics 2017;140(6):pii: e20163947.
Folschweiller 2019 {published data only}
-
- Folschweiller N, Behre U, Dionne M, Durando P, Esposito S, Ferguson L, et al. Long-term cross-reactivity against nonvaccine human papillomavirus types 31 and 45 after 2- or 3-dose schedules of the AS04-adjuvanted human HPV-16/18 vaccine. Journal of Infectious Diseases 2019;219(11):1799-803.
Garland 2007 {published data only}
-
- Garland SM, Steben M, Hernandez-Avila M, Koutsky LA, Wheeler CM, Perez G, et al. Noninferiority of antibody response to human papillomavirus type 16 in subjects vaccinated with monovalent and quadrivalent L1 virus-like particle vaccines. Clinical and Vaccine Immunology 2007;14(6):792-5.
Giacomet 2014 {published data only}
-
- Giacomet V, Penagini F, Trabattoni D, Vigano A, Rainone V, Bernazzani G, et al. Safety and immunogenicity of a quadrivalent human papillomavirus vaccine in HIV-infected and HIV-negative adolescents and young adults. Vaccine 2014;32(43):5657-61.
Giuliano 2007 {published data only}
-
- Giuliano AR, Lazcano-Ponce E, Villa L, Nolan T, Marchant C, Radley D, et al. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. Journal of Infectious Diseases 2007;196(8):1153-62.
Godi 2019 {published data only}
-
- Godi A, Panwar K, Haque M, Cocuzza CE, Andrews N, Southern J, et al. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine 2019;37(18):2455-62.
Harro 2001 {published data only}
-
- Harro CD, Pang YS, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. Journal of the National Cancer Institute 2001;93(4):284-92.
Hillman 2012 {published data only}
-
- Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Vardas E, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clinical and Vaccine Immunology 2012;19(2):261-7.
Hu 2022 {published data only}
Kosalaraksa 2015 {published data only}
-
- Kosalaraksa P, Mehlsen J, Vesikari T, Forsten A, Helm K, Van Damme P, et al. An open-label, randomized study of a 9-valent human papillomavirus vaccine given concomitantly with diphtheria, tetanus, pertussis and poliomyelitis vaccines to healthy adolescents 11-15 years of age. Pediatric Infectious Disease Journal 2015;34(6):627-34.
Koutsky 2002 {published data only}V501‐005
-
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB. A controlled trial of a human papillomavirus type 16 vaccine. New England Journal of Medicine 2002;347(21):1645-51.
-
- Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstetrics and Gynecology 2006;107(1):18-27.
-
- Rowhani-Rahbar A, Mao C, Hughes JP, Alvarez FB, Bryan JT, Hawes SE. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009;27(41):5612-9.
Krajden 2011 {published data only}
-
- Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clinical and Vaccine Immunology 2011;18(3):418-23.
Krajden 2014 {published data only}
-
- Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, et al. Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine 2014;32(5):624-30.
Kreimer 2015 {published data only}
-
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncology 2015;16(7):775-86.
Lamontagne 2013 {published data only}
-
- Lamontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. Journal of Infectious Diseases 2013;208(8):1325-34.
Lazcano‐Ponce 2018 {published data only}
-
- Lazcano-Ponce E, Carnalla-Cortes M, Barrientos-Gutierrez T, Torres-Ibarra L, Cruz-Valdez A, Salmeron J, et al. The effect of a booster dose of HPV tetravalent vaccine after 51 months: implications for extended vaccination schedules. Salud Publica de Mexico 2018;60(6):666-73.
Lehtinen 2017 {published data only}
-
- Lehtinen M, Lagheden C, Luostarinen T, Eriksson T, Apter D, Harjula K, et al. Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials. BMJ Open 2017;7(8):e015867.
Lin 2014 {published data only}
-
- Lin CJ, Zimmerman RK, Nowalk MP, Huang HH, Raviotta JM. Randomized controlled trial of two dosing schedules for human papillomavirus vaccination among college-age males. Vaccine 2014;32(6):693-9.
Markowitz 2023 {published data only}
-
- Markowitz LE, Unger ER. Human papillomavirus vaccination. New England Journal of Medicine 2023;388(19):1790-8. [DOI: 10.1056/NEJMcp2108502] - DOI
Neuzil 2011 {published data only}
-
- Neuzil KM, Canh DG, Thiem VD, Janmohamed A, Huong VM, Tang Y, et al. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. Journal of the American Medical Association 2011;305(14):1424-32.
Nygard 2015 {published data only}
-
- Nygard M, Saah A, Munk C, Tryggvadottir L, Enerly E, Hortlund M, et al. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clinical and Vaccine Immunology 2015;22(8):943-8.
Ogilvie 2017 {published data only}
-
- Ogilvie G, Sauvageau C, Dionne M, McNeil S, Krajden M, Money D, et al. Immunogenicity of 2 vs 3 doses of the quadrivalent human papillomavirus vaccine in girls aged 9 to 13 years after 60 months. JAMA 2017;317(16):1687-8.
Olsson 2007 {published data only}
-
- Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007;25(26):4931-9.
Olsson 2020 {published data only}
-
- Olsson SE, Restrepo JA, Reina JC, Pitisuttithum P, Ulied A, Varman M, et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: interim analysis after 8 years of follow-up. Papillomavirus Research 2020;10:100203.
Panagiotou 2015 {published data only}
-
- Panagiotou OA, Befano BL, Gonzalez P, Rodriguez AC, Herrero R, Schiller JT, et al. Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV vaccine trial. BMJ 2015;351:h4358.
Petaja 2011 {published data only}
-
- Petaja T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, et al. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. International Journal of Cancer 2011;129(9):2147-57.
Petersen 2017 {published data only}
-
- Petersen LK, Restrepo J, Moreira ED, Iversen O-E, Pitisuttithum P, Van Damme P, et al. Impact of baseline covariates on the immunogenicity of the 9-valent HPV vaccine - a combined analysis of five phase III clinical trials. Papillomavirus Research 2017;3:105‐15.
Puthanakit 2016 {published data only}
-
- Huang LM, Puthanakit T, Cheng-Hsun C, Ren-Bin T, Schwarz T, Pellegrino A, et al. Sustained immunogenicity of 2-dose human papillomavirus 16/18 AS04-adjuvanted vaccine schedules in girls aged 9-14 years: a randomized trial. Journal of Infectious Diseases 2017;215(11):1711-9.
-
- Puthanakit T, Huang LM, Chiu CH, Tang RB, Schwarz TF, Esposito S, et al. Randomized open trial comparing 2-dose regimens of the human papillomavirus 16/18 AS04-adjuvanted vaccine in girls aged 9-14 years versus a 3-dose regimen in women aged 15-25 years. Journal of Infectious Diseases 2016;214(4):525-36.
Rowhani‐Rahbar 2012 {published data only}
-
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, Hughes JP, Hawes SE, Weiss NS, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. Journal of Clinical Virology 2012;53(3):239-43.
Safaeian 2013a {published data only}
-
- Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: results from the Costa Rica Vaccine Trial. Human Vaccines & Immunotherapeutics 2013;9(7):1399-406.
Safaeian 2013b {published data only}
-
- Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prevention Research (Philadelphia, Pa.) 2013;6(11):1242-50.
Sankaranarayanan 2018 {published data only}
-
- Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018;36(32 Pt A):4783-91.
Schilling 2015 {published data only}
-
- Schilling A, Parra MM, Gutierrez M, Restrepo J, Ucros S, Herrera T, et al. Coadministration of a 9-valent human papillomavirus vaccine with meningococcal and tdap vaccines. Pediatrics 2015;136(3):e563-572.
Schwarz 2017 {published data only}
-
- Schwarz TF, Galaj A, Spaczynski M, Wysocki J, Kaufmann AM, Poncelet S, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Medicine 2017;6(11):2723-31.
Schwarz 2019 {published data only}
-
- Schwarz TF, Huang LM, Valencia A, Panzer F, Chiu CH, Decreux A, et al. A ten-year study of immunogenicity and safety of the AS04-HPV-16/18 vaccine in adolescent girls aged 10-14 years. Human Vaccines and Immunotherapeutics 2019;15(7-8):1970-9.
Watson‐Jones 2012 {published data only}
-
- Watson-Jones D, Baisley K, Ponsiano R, Lemme F, Remes P, Ross D, et al. Human papillomavirus vaccination in Tanzanian schoolgirls: cluster-randomized trial comparing 2 vaccine-delivery strategies. Journal of Infectious Diseases 2012;206(5):678-86.
Wheeler 2011 {published data only}
-
- Wheeler CM, Harvey BM, Pichichero ME, Simon MW, Combs SP, Blatter MM, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatric Infectious Disease Journal 2011;30(12):e225-e234.
Yu 2020 {published data only}
-
- Yu X-J, Li J, Lin Z-J, Zhao H, Lin B-Z, Qiao Y-L, et al. Immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine under different vaccination intervals. Human Vaccines and Immunotherapeutics 2020;16(7):1630-5.
Zimmerman 2010 {published data only}
-
- Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko F-S, Wettick E, et al. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college-aged women. Journal of Women's Health 2010;19(8):1441-7.
References to studies awaiting assessment
4v‐Cecolin Zaman 2024‐BGD/GHA {published and unpublished data}PACTR202008675647876
-
- NCT04508309. Phase 3 trial of a bivalent HPV vaccine (Cecolin®) in young girls. https://clinicaltrials.gov/show/NCT04508309 (accessed on 06 October 2022).
-
- Zaman K, Schuind AE, Adjei S, Antony K, Aponte JJ, Buabeng PB, et al. Safety and immunogenicity of Innovax bivalent human papillomavirus vaccine in girls 9-14 years of age: interim analysis from a phase 3 clinical trial. Vaccine 2024;42(9):2290-8.
9v Berenson 2024‐USA {published data only}
9v MacCosham 2022‐CAN {published data only}
-
- MacCosham A, El-Zein M, Burchell AN, Tellier P-P, Coutlee F, Franco EL. Transmission reduction and prevention with HPV vaccination (TRAP-HPV) study protocol: a randomised controlled trial of the efficacy of HPV vaccination in preventing transmission of HPV infection in heterosexual couples. BMJ Open 2020;10:e039383.
-
- MacCosham A, El-Zein M, Burchell AN, Tellier PP, Coutlée F, Franco EL, TRAP-HPV study group. Protection to self and to one's sexual partner after human papillomavirus vaccination: preliminary analysis from the Transmission Reduction and Prevention with HPV Vaccination study. Sexually Transmitted Diseases 2022;49(6):414-22.
Barnabas 2023 {published data only}
HPV‐003 {unpublished data only}HPV‐003
-
- GlaxoSmithKline. Clinical study report: a Phase I/II study to evaluate the safety and immunogenicity of MEDI-517, a virus-like particle vaccine against human papillomavirus (HPV) types 16 and 18, in healthy adult female volunteers who are HPV-16 or HPV-18 DNA positive. https://www.gsk-studyregister.com/en/trial-details/?id=580299/003 (accessed on 06 October 2022).
NCT00520598 {unpublished data only}V505‐001
-
- NCT00520598. Broad spectrum HPV (human papillomavirus) vaccine in 16 to 26 year old women (V505-001). https://clinicaltrials.gov/show/NCT00520598. https://clinicaltrials.gov/show/NCT00520598 (accessed on 06 October 2022).
NCT05149248 {unpublished data only}
-
- NCT05149248. Prevention and control of neoplasms associated with HPV in high-risk groups in Mexico City: the Condesa Study. https://clinicaltrials.gov/show/NCT05149248 (accessed on 06 October 2022).
Rivera 2018 {published data only}
-
- NCT01755689. Immunogenicity and safety study of glaxosmithkline (GSK) biologicals' meningococcal vaccine with or without co-administration of Cervarix and Boostrix in female adolescents and young adults. https://clinicaltrials.gov/ct2/show/results/NCT01755689 (accessed on 20 April 2023).
-
- Rivera L, Chanthavanich P, Poder A, Suryakiran PV, Jastorff A, Van der Wielen M. MenACWY-TT is immunogenic when co-administered with Tdap and AS04-HPV16/18 in girls and young women: results from a phase III randomized trial. Vaccine 2018;36(27):3967-75.
Shing 2022 {published data only}
-
- Shing JZ, Hu S, Herrero R, Hildesheim A, Porras C, Sampson JN, et al. Precancerous cervical lesions caused by non-vaccine-preventable HPV types after vaccination with the bivalent AS04-adjuvanted HPV vaccine: an analysis of the long-term follow-up study from the randomised Costa Rica HPV Vaccine Trial. Lancet Oncology 2022;23(7):940-9. [DOI: 10.1016/S1470-2045(22)00291-1] [PMID: ] - DOI - PMC - PubMed
V503‐018 {unpublished data only}
-
- Food and Drug Administration. Clinical Review of Biologics License Application Supplement STN# 125126/1297.0 – male indication for GARDASIL. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedPro... 2009.
Watson‐Jones 2023 {published data only}
-
- Watson-Jones D, Changalucha J, Whitworth H, Pinto L, Mutani P, Indangasi J, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. Obstetrical & Gynecological Survey 2023;78:154.
Yao 2022 {published data only}
Zhao 2022a {published data only}
-
- Zhao C, Zhao Y, Li J, Li M, Su Y, Mi X, et al. The eight-year long-term follow-up on the effectiveness of the quadrivalent human papillomavirus vaccine in Chinese women 20-45 years of age. Human Vaccines & Immunotherapeutics 2022;18(5):2052700. [DOI: 10.1080/21645515.2022.2052700] [PMID: ] - DOI - PMC - PubMed
Zhao 2022b {published data only}
-
- Zhao FH, Wu T, Hu YM, Wei LH, Li MQ, Huang WJ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Human Papillomavirus (16 and 18) L1 virus-like-particle vaccine: end-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infectious Diseases 2022;22(12):1756-68. [DOI: 10.1016/S1473-3099(22)00435-2] [PMID: ] - DOI - PubMed
Zhao 2023 {published data only}
-
- Zhao F, Jastorff A, Hong Y, Hu S, Chen W, Xu X, et al. Safety of AS04-HPV-16/18 vaccine in Chinese women aged 26 years and older and long-term protective effect in women vaccinated at age 18-25 years: a 10-year follow-up study. Asia-Pacific Journal of Clinical Oncology 2023;19(4):458-67. [DOI: 10.1111/ajco.13833] [PMID: ] - DOI - PubMed
Zhong 2023 {published data only}
-
- Zhong G, Zhuang C, Hu X, Chen Q, Bi Z, Jia X, at al. Safety of hepatitis E vaccination for pregnancy: a post-hoc analysis of a randomized, double-blind, controlled phase 3 clinical trial. Emerging Microbes & Infections 2023;12(1):2185456. [DOI: 10.1080/22221751.2023.2185456] [PMID: ] - DOI - PMC - PubMed
References to ongoing studies
Add‐Vacc {published data only}
-
- NCT04953130. Adding male single dose HPV vaccination to female HPV vaccination in Tanzania. https://clinicaltrials.gov/show/NCT04953130 (accessed 06 October 2022).
ESCUDDO {published data only}
-
- Porras C, Sampson JN, Herrero R, Gail MH, Cortes B, Hildesheim A, et al. Rationale and design of a double-blind randomized non-inferiority clinical trial to evaluate one or two doses of vaccine against human papillomavirus including an epidemiologic survey to estimate vaccine efficacy: the Costa Rica ESCUDDO trial. Vaccine 2022;40:76-88.
Giuliano 2022 {published data only}EUCTR2019‐003236‐23V503‐049
-
- Giuliano AR, Wilkin T, Bautista OM, Cheon K, Connor L, Dubey S, et al. Design of a Phase III efficacy, immunogenicity, and safety study of 9-valent human papillomavirus vaccine in prevention of oral persistent infection in men. Contemporary Clinical Trials 2022;115:106592.
HANDS {unpublished data only}
-
- NCT03832049. HPV vaccination in Africa - new delivery schedules alias the HANDS HPV vaccine trial. https://clinicaltrials.gov/show/NCT03832049 (accessed 06 October 2022).
ICI‐VPH {unpublished data only}
-
- NCT02009800. ICI-VPH: impact of HPV immunisation schedules against HPV. https://clinicaltrials.gov/show/NCT02009800 (accessed 06 October 2022).
NCT03943875 {unpublished data only}
-
- NCT03943875. GARDASIL 9: 3 dose vs. 2 dose with delayed 3rd dose. https://clinicaltrials.gov/show/NCT03943875 (accessed 06 October 2022).
NCT03998254 {unpublished data only}V503‐023
-
- NCT03998254. Efficacy, immunogenicity and safety of V503 in Chinese women aged 20–45 years (V503-023). https://clinicaltrials.gov/show/NCT03998254 (accessed 06 October 2022).
NCT04635423 {unpublished data only}EUCTR2020‐001047‐67jRCT2031200217V503‐064
-
- NCT04635423. Efficacy, immunogenicity, and safety study of the 9vHPV vaccine in Japanese males (V503-064). https://clinicaltrials.gov/show/NCT04635423 (accessed 06 October 2022).
NCT04772534 {unpublished data only}EUCTR2020‐001170‐29V503‐066
-
- NCT04772534. Immunogenicity and safety of the 9-valent human papillomavirus (9vHPV) vaccine in Japanese boys and girls (V503-066). https://clinicaltrials.gov/show/NCT04772534 (accessed 06 October 2022).
NCT05279248 {unpublished data only}
-
- NCT05279248. The immunogenicity and safety of the vaccination of human papillomavirus vaccine and measles-mumps-rubella vaccine. https://clinicaltrials.gov/study/NCT05279248 (accessed 07 November 2024).
NCT05415345 {unpublished data only}
-
- NCT05415345. Immunogenicity and safety of co-immunization with Cecolin and Hecolin. https://clinicaltrials.gov/study/NCT05415345 (accessed 07 November 2024).
NCT05672927 {unpublished data only}
-
- NCT05672927. Comparing immune response of 2 vs 3 HPV doses (27-45 years old). https://clinicaltrials.gov/study/NCT05672927 (accessed 07 November 2024).
NCT06345885 {unpublished data only}ChiCTR2300069209
-
- ChiCTR2300069209. Study on the safety and immunogenicity of the immunization procedure for one dose of HPV vaccine. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2300069209 (accessed 07 November 2024).
-
- NCT06345885. Immunogenicity and safety of one dose of HPV vaccine. https://clinicaltrials.gov/study/NCT06345885 (accessed 07 November 2024).
PRISMA ESCUDDO {unpublished data only}
-
- NCT05237947. Single-dose HPV vaccination for the prevention of cervical cancer in young adult women in Costa Rica, the PRISMA ESCUDDO trial (PRISMA). https://clinicaltrials.gov/study/NCT05237947 (accessed 07 November 2024).
Salmeron 2016 {published data only}
-
- Salmeron J, Torres-Ibarra L, Bosch FX, Cuzick J, Lorincz A, Wheeler CM, et al. HPV vaccination impact on a cervical cancer screening program: methods of the FASTER-Tlalpan Study in Mexico. Salud Publica de Mexico 2016;58:211-9.
Additional references
Adhanom‐Ghebreyesus 2018
-
- Adhanom-Ghebreyesus T. Cervical Cancer: An NCD We Can Overcome. World Health Organization (WHO) 2018 [cited 19 May 2018]. Available from: https://www.who.int/dg/speeches/detail/cervical-cancer-an-ncd-we-can-ove... (accessed prior to 29 October 2025).
Arbyn 2018
-
- Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD009069. [DOI: 10.1002/14651858.CD009069.pub3] - DOI
Bergman 2019
-
- Bergman H, Buckley BS, Villanueva G, Petkovich J, Garrity C, Lutje V, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013479. [DOI: 10.1002/14651858.CD013479] - DOI
Bi 2020
-
- Bi D, Apter D, Eriksson T, Hokkanen M, Zima J, Damaso S, et al. Safety of the AS04-adjuvanted human papillomavirus (HPV)-16/18 vaccine in adolescents aged 12-15 years: end-of-study results from a community-randomized study up to 6.5 years. Human Vaccines and Immunotherapeutics 2020;16(6):1392-403.
Bleeker 2009
-
- Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer C. Penile cancer: epidemiology, pathogenesis and prevention. World Journal of Urology 2009;27:141-50.
Block 2006
-
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006;118(5):2135-45.
Bosch 2002
-
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology 2002;55:244-65.
Bray 2018
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 2018;68(6):394-424.
Buttmann‐Schweiger 2015
-
- Buttmann-Schweiger N, Klug SJ, Luyten A, Holleczek B, Heitz F, Du Bois A, et al. Incidence patterns and temporal trends of invasive nonmelanotic vulvar tumors in Germany 1999‑2011: a population-based cancer registry analysis. PloS One 2015;10(5):e0128073.
Campbell 1989
-
- Campbell AV. A report from New Zealand: an "unfortunate experiment". Bioethics 1989;3(1):59-66.
Cancer Research UK 2021a
-
- Cancer Research UK. Cervical cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed 15 December 2021).
Cancer Research UK 2021b
-
- Cancer Research UK. Cervical cancer incidence by stage at diagnosis. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed 15 December 2021).
Cancer Research UK 2025a
-
- Cancer Research UK. Penile cancer incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed prior to 29 October 2025).
Cancer Research UK 2025b
-
- Cancer Research UK. Anal cancer incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed prior to 29 October 2025).
Cancer Research UK 2025c
-
- Cancer Research UK. Head and neck cancers incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed prior to 29 October 2025).
Cancer Research UK 2025d
-
- Cancer Research UK. Vaginal cancer incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed prior to 29 October 2025).
Cancer Research UK 2025e
-
- Cancer Research UK. Vulval cancer incidence. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed prior to 29 October 2025).
Castanon 2018
-
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modelling study. Lancet. Public Health 2018;3(1):e34-e43.
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synththesis Methods 2012;3(2):161-76.
Cochrane Library 2020
-
- Cochrane Library. Conflict of interest policy for Cochrane Library content, Version 3.6 (2020). https://training.cochrane.org/online-learning/coi-policy/coi-policy-coch... (accessed 11 December 2024).
Corcoran 2018
-
- Corcoran B, Clarke A, Barrett T. Rapid response to HPV vaccination crisis in Ireland. Lancet 2018;391(10135):2103.
D'Addario 2017
-
- D'Addario M, Redmond S, Scott P, Egli-Gany D, Riveros-Balta AX, Henao Restrepo AM, et al. Two-dose schedules for human papillomavirus vaccine: systematic review and meta-analysis. Vaccine 2017;35(22):2892-901.
Daling 2002
Daling 2004
-
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004;101(2):270-80. [DOI: 10.1002/cncr.20365] - DOI
Dareng 2019
-
- Dareng EO, Adebamowo SN, Famooto A, Olawande O, Odutola MK, Olaniyan Y, et al. Prevalence and incidence of genital warts and cervical human papillomavirus infections in Nigerian women. BMC Infectious Diseases 2019;19(1):27-36. [DOI: ]
De Angelis 2014
-
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5 - a population-based study. Lancet Oncology 2014;15(1):23-34.
De Martel 2017
-
- De Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. International Journal of Cancer 2017;141(4):664-70.
Desai 2011
-
- Desai S, Wetten S, Woodhall SC, Peters L, Hughes G, Soldan K. Genital warts and cost of care in England. Sexually Transmitted Infections 2011;87(6):464.
Deshmukh 2020
-
- Deshmukh AA, Suk R, Shiels MS, Sonawane K, Nyitray AG, Liu Y, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001–2015. Journal of the National Cancer Institute 2020;112(8):829-38. [DOI: 10.1093/jnci/djz219] - DOI
DistillerSR 2021 [Computer program]
-
- DistillerSR. Version 2.35. Evidence Partners, 2021. Available from: https://www.evidencepartners.com.
Dobson 2013
-
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309(17):1793-802.
D’Souza 2017
-
- D’Souza G, Westra WH, Wang SJ, Van Zante A, Wentz A, Kluz N, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncology 2017;3(2):169-77. [DOI: 10.1001/jamaoncol.2016.3067] - DOI
Falcaro 2021
-
- Falcaro M, Castanon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 2021;398(10316):2084-92. [DOI: 10.1016/S0140-6736(21)02178-4] - DOI
FDA 2022
-
- Food and Drug Administration. What is a serious adverse event? https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-a... (accessed 26 January 2022).
Ferlay 2020a
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Vagina fact sheet. Global Cancer Observatory: Cancer Today. 2020. Available at https://gco.iarc.who.int/media/globocan/factsheets/cancers/22-vagina-fac... (accessed prior to 29 October 2025).
Ferlay 2020b
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Penis fact sheet. Global Cancer Observatory: Cancer Today. 2020. Available at https://gco.iarc.who.int/media/globocan/factsheets/cancers/26-penis-fact... (accessed prior to 29 October 2025).
Ferlay 2020c
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Anus fact sheet. Global Cancer Observatory: Cancer Today. 2020. Available at https://gco.iarc.who.int/media/globocan/factsheets/cancers/10-anus-fact-... (accessed prior to 29 October 2025).
Ferlay 2020d
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Vulva fact sheet. Global Cancer Observatory: Cancer Today. 2020. Available at https://gco.iarc.who.int/media/globocan/factsheets/cancers/21-vulva-fact... (accessed prior to 29 October 2025).
Fu 2022
Gallagher 2018
-
- Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018;36(32):4761-7.
Goodman 2010
-
- Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, et al. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV cohort study. Journal of Infectious Diseases 2010;201(9):1331-9. [DOI: 10.1086/651620] - DOI
GSK 2023
-
- GSK. GSK Study Register. https://www.gsk-studyregister.com/en/ (accessed 20 April 2023).
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94.
Hawkins 2013
-
- Hawkins MG, Winder DM, Ball SL, Vaughan K, Sonnex C, Stanley MA, et al. Detection of specific HPV subtypes responsible for the pathogenesis of condylomata acuminata. Virology Journal 2013;10:137. [DOI: 10.1186/1743-422X-10-137] - DOI
Henschke 2022
-
- Henschke N, Bergman H, Buckley B, et al. Efficacy, effectiveness and immunogenicity of one dose of HPV vaccine compared with no vaccination, 2 doses, or 3 doses. Cochrane Response systematic review presented to WHO SAGE. Virtual meeting, WHO Geneva, Switzerland; 4-7 April 2022. [URL: https://terrance.who.int/mediacentre/data/sage/SAGE_Slidedeck_Apr2022.pdf]
Henschke 2025
-
- Henschke N, Bergman H, Villanueva G, Loke YK, Golder SP, Crosbie EJ, et al. Effects of human papillomavirus (HPV) vaccination programmes on community rates of HPV-related disease and harms from vaccination. Cochrane Database of Systematic Reviews 2025, Issue 11. Art. No: CD015363. [DOI: 10.1002/14651858.CD015363.pub2] - DOI
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2022
-
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook..
Hviid 2020
-
- Hviid A, Thorsen NM, Valentiner-Branth P, Frisch M, Molbak K. Association between quadrivalent human papillomavirus vaccination and selected syndromes with autonomic dysfunction in Danish females: population based, self-controlled, case series analysis. BMJ (Clinical Research Ed.) 2020;370:m2930.
IARC 2014
-
- International Agency for Research in Cancer. Primary End-Points for Prophylactic HPV Vaccine Trials. IARC Working Group Report. World Health Organization International Agency for Research on Cancer, 2014.
Insinga 2005
-
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. PharmacoEconomics 2005;23(11):1107-22.
Insinga 2011
-
- Insinga RP, Perez G, Wheeler CM, Koutsky LA, Garland SM, Leodolter S, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiology Biomarkers & Prevention 2011;20(2):287-96.
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781-8.
Jaisamrarn 2013
-
- Jaisamrarn U, Castellsague X, Garland SM, Naud P, Palmroth J, Del Rosario-Raymundo MR, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PloS One 2013;8(11):e79260.
JCVI 2022
-
- Joint Committee on Vaccination and Immunisation (JCVI) Department of Health and Social Care, London, UK. JCVI statement on a one-dose schedule for the routine HPV immunisation programme; 5 August 2022. Available at https://www.gov.uk/government/publications/single-dose-of-hpv-vaccine-jc... (accessed prior to 29 October 2025).
Jørgensen 2018a
-
- Jørgensen L, Gøtzsche PC, Jefferson T. The Cochrane HPV vaccine review was incomplete and ignored important evidence of bias. BMJ Evidence Based Medicine 2018;23(5):165-8.
Jørgensen 2020a
-
- Jørgensen L, Gøtzsche PC, Jefferson T. Benefits and harms of the human papillomavirus (HPV) vaccines: comparison of trial data from clinical study reports with corresponding trial register entries and journal publications. Systematic Reviews 2020;9(1):42.
Jørgensen 2020b
-
- Jørgensen L, Gøtzsche PC, Jefferson T. Benefits and harms of the human papillomavirus (HPV) vaccines: systematic review with meta-analyses of trial data from clinical study reports. Systematic Reviews 2020;9(1):43.
Jørgensen 2018b
-
- Jørgensen L, Doshi P, Gøtzsche P, Jefferson T. Challenges of independent assessment of potential harms of HPV vaccines. BMJ (Clinical Research Ed.) 2018;362:k3694.
Jørgensen 2018c
-
- Jørgensen L, Gøtzsche PC, Jefferson T. Index of the human papillomavirus (HPV) vaccine industry clinical study programmes and non-industry funded studies: a necessary basis to address reporting bias in a systematic review. Systematic Reviews 2018;7(1):8.
Kang 2017
-
- Kang Y, Smith M, Barlow E, Coffey K, Hacker Neville, Canfell K. Vulvar cancer in high-income countries: Increasing burden of disease. International Journal of Cancer 2017;141(11):2174-86.
Karafillakis 2019
-
- Karafillakis E, Simas C, Jarrett C, Verger P, Peretti-Watel P, Dib F, et al. HPV vaccination in a context of public mistrust and uncertainty: a systematic literature review of determinants of HPV vaccine hesitancy in Europe. Human Vaccines and Immunotherapeutics 2019;15(7-8):1615-27.
Kirkham 2018
-
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ (Clinical Research Ed.) 2018;362:k3802.
Kirnbauer 1992
-
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proceedings of the National Academy of Sciences 1992;89(24):12180-4. [DOI: 10.1073/pnas.89.24.12180] - DOI
Kjaer 2021
-
- Kjaer SK, Dehlendorff C, Belmonte F, Baandrup L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. Journal of the National Cancer Institute 2021;113(10):1329-35. [DOI: 10.1093/jnci/djab080] - DOI
Koliopoulos 2017
-
- Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin‐Hirsch PP, Mustafa RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD008587. [DOI: 10.1002/14651858.CD008587.pub2] - DOI
Kreimer 2011
-
- Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. Journal of the National Cancer Institute 2011;103(19):1444-51.
Kreimer 2015
-
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncology 2015;16(7):775-86.
Kyrgiou 2017
-
- Kyrgiou M, Athanasiou A, Kalliala IE, Paraskevaidi M, Mitra A, Martin-Hirsch PP, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012847. [DOI: 10.1002/14651858.CD012847] - DOI
Lacey 2019
-
- Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Research 2019;8:100174.
Lai 2014
-
- Lai J, Elleray R, Nordin A, Hirschowitz L, Rous B, Gildea C, et al. Vulval cancer incidence, mortality and survival in England: age-related trends. BJOG 2014;121(6):728-38; discussion 39.
LaMontagne 2017
-
- LaMontagne DS, Bloem PJ, Brotherton JM, Gallagher KE, Badiane O, Ndiaye C. Progress in HPV vaccination in low- and lower-middle-income countries. International Journal of Gynaecology and Obstetrics 2017;138:7-14.
Landy 2018
-
- Landy R, Windridge P, Gillman MS, Sasieni PD. What cervical screening is appropriate for women who have been vaccinated against high risk HPV? A simulation study. International Journal of Cancer 2018;142(4):709-18.
Leemans 2018
-
- Leemans CR, Snijders PJ, Brakenhoff RH. The molecular landscape of head and neck cancer. Nature Reviews Cancer 2018;18(5):269-82.
Lei 2020
-
- Lei J, Ploner A, Elfstrom KM, Wang J, Roth A, Fang F, et al. HPV vaccination and the risk of invasive cervical cancer. New England Journal of Medicine 2020;383(14):1340-8.
Lineberry 2016
-
- Lineberry N, Berlin JA, Mansi B, Glasser S, Berkwits M, Klem C, et al. Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective. BMJ (Clinical Research Ed.) 2016;355:i5078.
Mannweiler 2013
-
- Mannweiler S, Sygulla S, Winter E, Regauer S. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. Journal of the American Academy of Dermatology 2013;69(1):73-81. [DOI: 10.1016/j.jaad.2012.12.973] - DOI
Markowitz 2018
-
- Markowitz LE, Drolet M, Perez N, Jit M, Brisson M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine 2018;36(32 Pt A):4806-15.
McCredie 2008
-
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncology 2008;9(5):425-34.
McIndoe 1984
-
- McIndoe WA, McLean MR, Jones RW, Mullins PR. The invasive potential of carcinoma in situ of the cervix. Obstetrics and Gynecology 1984;64(4):451-8.
Mignozzi 2024
-
- Mignozzi S, Santucci C, Malvezzi M, Levi F, La Vecchia C, Negri E. Global trends in anal cancer incidence and mortality. European Journal of Cancer Prevention 2024;33(2):77-86.
Morrison 2024
-
- Morrison J, Baldwin P, Hanna L, Andreou A, Buckley L, Durrant L, et al. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: an update on recommendations for practice 2023. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2024;292:210-38. [DOI: 10.1016/j.ejogrb.2023.11.013] [PMID: ] - DOI - PubMed
Munoz 1996
-
- Munoz N, Bosch FX. The causal link between HPV and cervical cancer and its implications for prevention of cervical cancer. Bulletin of the Pan American Health Organization 1996;30(4):362-77.
Nasser 2023
-
- Nasser S, Togo PA, Elghanmi A, BIlir E, Sehouli J. Call for implementation research: cross-continental partnership experiences from the Trans-African Digital e-Health Network (i-STARC Project). International Journal of Gynecologic Cancer 2023;33:1469-70.
NHS Digital 2020a
-
- NHS Digital. Cervical Screening Programme, England - 2019-20: Official statistics, National statistics; November 2020. Available at https://digital.nhs.uk/data-and-information/publications/statistical/cer... (accessed prior to 29 October 2025).
NHS Digital 2020b
-
- NHS Digital. Cervical Screening Programme, England - 2019-20: Official statistics, National statistics: Section 3: Colposcopy; November 2020. Available at https://digital.nhs.uk/data-and-information/publications/statistical/cer... (accessed prior to 29 October 2025).
Page 2021
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available at https://www.cochrane.org/authors/handbooks-and-manuals/handbook/archive/....
Patel 2013
Peto 2004
-
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364(9430):249-56.
Pils 2017
-
- Pils S, Gensthaler L, Alemany L, Horvat R, Sanjose S, Joura EA. HPV prevalence in vulvar cancer in Austria. Wiener Klinische Wochenschrift 2017;129(21-22):805-9.
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical Research Ed.) 2014;349:g5630.
Qiao 2020
-
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent Human papillomavirus vaccine: an interim analysis of a randomized clinical trial. Journal of the National Cancer Institute 2020;112(2):145-53.
RevMan 2025 [Computer program]
-
- Review Manager (RevMan). Version 9.14.0. The Cochrane Collaboration, 2025. Available at https://revman.cochrane.org.
Rickford 2023
-
- Rickford R, Rogers M, Halliday A, Lamptey P, Kola-Palmer S. Attitudes to reducing cervical screening frequency among UK women: a qualitative analysis. Psycho-oncology 2023;32(5):721-9.
Sabatini 2020
-
- Sabatini ME, Chiocca S. Human papillomavirus as a driver of head and neck cancers. British Journal of Cancer 2020;122(3):306-14. [DOI: 10.1038/s41416-019-0602-7] - DOI
Sankaranarayanan 2016
-
- Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncology 2016;17(1):67-77.
SEER 2024
-
- National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Anal Cancer. Available at https://seer.cancer.gov/statfacts/html/anus.html (accessed February 2024).
Singh 2023
-
- Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Global Health 2023;11(2):e197-e206.
Sonnenberg 2019
-
- Sonnenberg P, Tanton C, Mesher D, King E, Beddows S, Field N, et al. Epidemiology of genital warts in the British population: implications for HPV vaccination programmes. Sexually Transmitted Infections 2019;95(5):386-90. [DOI: 10.1136/sextrans-2018-053786] - DOI
Stanley 2006
-
- Stanley MA. Human papillomavirus vaccines. Reviews in Medical Virology 2006;16:139-49.
Stata 2017 [Computer program]
-
- StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC, 2017.
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002.
Sterne 2019
-
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2019;366:l4898.
Sung 2021
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2021;71(3):209-49. [DOI: 10.3322/caac.21660] - DOI
Suppli 2018
-
- Suppli CH, Hansen ND, Rasmussen M, Valentiner-Branth P, Krause TG, Mølbak K. Decline in HPV-vaccination uptake in Denmark - the association between HPV-related media coverage and HPV-vaccination. BMC Public Health 2018;18(1):1360.
Thomas 2021
-
- Thomas J, McDonald S, Noel-Storr A, Shemlit I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2021;133:140-51.
Thomsen 2020
-
- Thomsen RW, Öztürk B, Pedersen L, Nicolaisen SK, Petersen I, Olsen J, et al. Hospital records of pain, fatigue, or circulatory symptoms in girls exposed to human papillomavirus vaccination: cohort, self-controlled case series, and population time trend studies. American Journal of Epidemiology 2020;189(4):277-85.
Tsai 2023
-
- Tsai SA, Lu CY, Chen TI, Huang SP, Chen YC. Adverse events from HPV vaccination in Taiwan. Vaccine 2023;41(49):7444-9.
Ujiie 2022
-
- Ujiie M, Kitano T, Tsuzuki S. Changing trends in HPV vaccination in Japan. Human Vaccines & Immunotherapeutics 2022;18(1):1-3.
Villa 2020
-
- Villa A, Patton LL, Giuliano AR, Estrich CG, Pahlke SC, O'Brien KK, et al. Summary of the evidence on the safety, efficacy, and effectiveness of human papillomavirus vaccines: Umbrella review of systematic reviews. Journal of the American Dental Association 2020;151(4):245-254.e24.
WHO 2017
-
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Weekly Epidemiological Record 2017;92:241-68.
WHO 2018
-
- World Health Organization. Cervical cancer. Available at http://www.who.int/cancer/prevention/diagnosis-screening/ cervical-cancer/en/ (accessed prior to 29 October 2025).
WHO 2019
-
- World Health Organization. WHO cervical cancer screening coverage data. Previously available at https://www.who.int/data/gho/data/indicators/indicator-details/GHO/cover... (accessed prior to 29 October 2025).
WHO 2021
-
- World Health Organization. List of prequalified vaccines. Available at https://extranet.who.int/pqweb/vaccines/list-prequalified-vaccines (accessed prior to 29 October 2025).
WHO 2022
-
- World Health Organization. WHO updates recommendations on HPV vaccination schedule; December 2022. Available at https://www.who.int/news/item/20-12-2022-WHO-updates-recommendations-on-... (accessed prior to 29 October 2025).
WHO 2023
-
- WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem; November 2020. Available at https://www.who.int/publications/i/item/9789240014107 (accessed 20 April 2023).
Willame 2016
-
- Willame C, Rosillon D, Zima J, Angelo MG, Stuurman AL, Vroling H, et al. Risk of new onset autoimmune disease in 9- to 25-year-old women exposed to human papillomavirus-16/18 AS04-adjuvanted vaccine in the United Kingdom. Human Vaccines and Immunotherapeutics 2016;12(11):2862-71.
Wong 2020
-
- Wong LP, Wong PF, Megat Hashim M, Han L, Lin Y, Hu Z, et al. Multidimensional social and cultural norms influencing HPV vaccine hesitancy in Asia. Human Vaccines and Immunotherapeutics 2020;16(7):1611-22.
Woodhall 2011
-
- Woodhall SC, Jit M, Soldan K, Kinghorn G, Gilson R, Nathan M, et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sexually Transmitted Infections 2011;87(6):458.
World Bank 2025
-
- World Bank Country and Lending Groups. Available at https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-b... (accessed prior to 29 October 2025).
Yepes‐Nuñez 2019
-
- Yepes-Nuñez JJ, Li S-A, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13.
Yoon 2021
-
- Yoon D, Lee JH, Lee H, Shin JY. Association between human papillomavirus vaccination and serious adverse events in South Korean adolescent girls: nationwide cohort study. BMJ (Clinical Research Ed.) 2021;372:m4931.
Zhan 2019
-
- Zhan Y, Liu X, Feng Y, Wu S, Yu J. Safety and efficacy of human papillomavirus vaccination for people living with HIV: a systematic review and meta-analysis. International Journal of STD & AIDS 2019;30(11):1105-15.
References to other published versions of this review
Bergman 2022
-
- Bergman H, Henschke N, Villanueva G, Loke YK, Golder SP, Dwan K, et al. Human papillomavirus (HPV) vaccination for the prevention of cervical cancer and other HPV‐related diseases: a network meta‐analysis. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD015364. [DOI: 10.1002/14651858.CD015364] - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

