Long-term mortality rate and clinical outcomes associated with femoro-popliteal drug-coated balloon angioplasty and drug-eluting stents in chronic limb-threatening ischaemia: an analysis of the BASIL-3 RCT
- PMID: 41276786
- PMCID: PMC12640773
- DOI: 10.1093/bjs/znaf251
Long-term mortality rate and clinical outcomes associated with femoro-popliteal drug-coated balloon angioplasty and drug-eluting stents in chronic limb-threatening ischaemia: an analysis of the BASIL-3 RCT
Abstract
Introduction: In recent years there have been a plethora of new endovascular devices have entered the market, including paclitaxel (PTX) drug-coated balloons (DCB) and drug-eluting stents (DES) for treating patients with chronic limb-threatening ischaemia (CLTI). There have been concerns that the use of PTX is associated with increased all-cause mortality rate in this patient population.
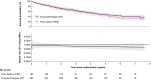
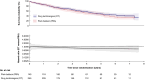
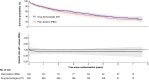
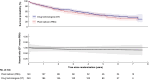
Methods: In the BASIL-3 trial (ISRCTN14469736) UK patients with CLTI were randomized (1:1:1) to receive femoro-popliteal (FP) plain balloon angioplasty (PBA; with or without bailout bare metal stenting (BMS), DCB angioplasty (DCBA) (with or without BMS), or primary DES. Here, data from the DCBA and DES arms have been pooled into a single 'drug technologies' (DT) group and compared with PBA ± BMS. The primary outcome was overall survival (OS). Secondary outcomes included amputation-free survival (AFS), major amputations, major adverse limb events, major adverse cardiovascular events, reinterventions, and 30-day mortality and morbidity rates.
Results: Four hundred and eighty-one participants were randomized (PBA: n = 160; DT: n = 321). At a median follow-up in survivors of 5.6 years, OS was similar between the pooled DT and PBA groups (adjusted hazard ratio (HR): 0.83; 95% c.i.: 0.64 to 1.07). There was no evidence of a statistically significant difference in AFS between the groups (adjusted HR: 0.84; 95% c.i.: 0.66 to 1.06), or other secondary outcomes.
Conclusions: This further pooled analysis of the BASIL-3 RCT does not support the notion that the use of drug-eluting technologies, when compared to plain balloon angioplasty, increases all-cause mortality rate, or has other clinically important adverse effects, when used in patients with CLTI.
Plain language summary
The BASIL-3 trial compared plain balloons with drug-coated ballons and drug-coated stents in a three-way comparison, which were used to stretch or reopen narrowed or blocked arteries in the leg below the groin. The main trial did not show any differences between these three devices in patients with chronic limb-threatening ischaemia. Due to concerns regarding an increased risk of death with drug-coated devices from other studies we have performed another analysis adding together both drug-coated balloons and stents into one group and comparing with the plain balloons and bare metal stents. This supplementary analysis did not show an increased risk of death with these drug devices in BASIL-3 participants.
© The Author(s) 2025. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures
References
-
- Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK et al. Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med 2022;387:2305–2316 - PubMed
-
- Bradbury AW, Moakes CA, Popplewell M, Meecham L, Bate GR, Kelly L et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet 2023;401:1798–1809 - PubMed
-
- Smith JA, So KL, Kashyap VS, Cho JS, Colvard B, Kumins NH. Outcome after revascularization with paclitaxel-coated devices in patients with chronic limb-threatening ischemia. J Vasc Surg 2023;77:1742–1750 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical