This is a preprint.
JAK1/2 Inhibition Delays Cachexia and Improves Survival through Increased Food Intake
- PMID: 41278737
- PMCID: PMC12633540
- DOI: 10.1101/2025.10.21.683287
JAK1/2 Inhibition Delays Cachexia and Improves Survival through Increased Food Intake
Abstract
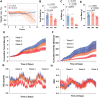
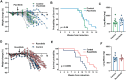
Lung cancer is the leading cause of cancer-related death and is frequently accompanied by reduced food intake and cachexia, a debilitating syndrome characterized by weight loss and skeletal muscle wasting. We sought to identify contributors to cachexia using a murine model of lung cancer that reproduces key features of this syndrome. A multiplex cytokine screening approach, integrated with western blot and transcriptomic analyses, identified tumor-derived inflammatory mediators and downstream signaling pathways associated with cachexia. Notably, IL-6 superfamily members were elevated in the tumor and plasma of mice and patients with cachexia. The JAK-STAT3 signaling was upregulated in liver and skeletal muscle, driving the acute phase response and impairing lipid metabolism. Pharmacologic inhibition of JAK1/2 with ruxolitinib improved body weight, fat mass, and overall survival without altering tumor burden. These effects were driven primarily by blunted hypothalamic leptin receptor signaling, which increased food intake early in the disease course. In the liver, JAK inhibition reduced STAT3 activity, restored fatty acid oxidation, and decreased the production of acute-phase proteins. These findings support JAK inhibition as a therapeutic strategy for lung cancer-associated cachexia.
Conflict of interest statement
Conflict of interest statement M.D.G. holds equity in Faeth Therapeutics and Skye Biosciences; reports consulting or advisory roles with Almac Discovery, Faeth Therapeutics, Genentech Inc., Scorpion Therapeutics, Skye Biosciences, and Third Arc Bio, Inc.; honoraria from Novartis AG, Pfizer Inc., Genentech Inc., patents, royalties, and other intellectual property with Weill Cornell Medicine and Faeth Therapeutics. E.D. reports intellectual property with Weill Cornell Medicine. All other authors report no conflicts.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
