This is a preprint.
Toll-like Receptor 4 Contributes to PCOS-like Metabolic and Reproductive Pathogenesis
- PMID: 41279684
- PMCID: PMC12632965
- DOI: 10.1101/2025.10.16.682907
Toll-like Receptor 4 Contributes to PCOS-like Metabolic and Reproductive Pathogenesis
Abstract
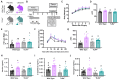
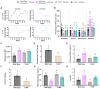
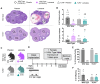
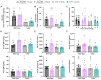
Polycystic ovary syndrome (PCOS) is a reproductive disorder with heterogeneous symptoms and severity. Despite extensive research documenting chronic immune dysfunction as a hallmark of PCOS, the specific molecular mechanisms driving immune activation and its connection to the syndrome's diverse symptoms remain poorly understood. Emerging evidence suggests that gut-derived bacterial endotoxins, particularly lipopolysaccharide (LPS), may breach the intestinal barriers in PCOS patients and trigger systemic inflammation through Toll-like receptor 4 (TLR4), a pattern recognition receptor of the innate immune system. This study investigated whether TLR4 serves as a critical mechanistic driver of PCOS pathogenesis by examining the effect of genetic TLR4 knockout (TLR4-/-) in a letrozole (LET)-induced mouse model of PCOS. Our results demonstrate that TLR4 deficiency reduces many PCOS-like symptoms, including elevated luteinizing hormone, anovulation, and metabolic dysfunction. TLR4 knockout also preserved estrous cycling and fertility, improved glucose tolerance, maintained gut barrier integrity, and reduced inflammatory markers in LET-treated females. These findings establish TLR4 as a key mediator orchestrating PCOS's multi-system pathology, positioning TLR4 as a critical convergence point rather than affecting individual symptoms in isolation. This novel work reveals that TLR4-mediated inflammation drives multiple PCOS pathologies, opening avenues for targeted anti-inflammatory treatments in women with this disorder.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures

References
-
- Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B. O., The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31, 2841–2855 (2016). - PubMed
-
- Kulkarni S., Gupta K., Ratre P., Mishra P. K., Singh Y., Biharee A., Thareja S., Polycystic ovary syndrome: Current scenario and future insights. Drug Discov. Today 28, 103821 (2023). - PubMed
-
- Unfer V., Kandaraki E., Pkhaladze L., Roseff S., Vazquez-Levin M. H., Laganà A. S., Shiao-Yng C., Yap-Garcia M. I. M., Greene N. D. E., Soulage C. O., Bevilacqua A., Benvenga S., Barbaro D., Pintaudi B., Wdowiak A., Aragona C., Kamenov Z., Appetecchia M., Porcaro G., Hernandez Marin I., Facchinetti F., Chiu T., Pustotina O., Papalou O., Nordio M., Cantelmi T., Cavalli P., Vucenik I., D’Anna R., Unfer V. R., Dinicola S., Salehpour S., Stringaro A., Montaninno Oliva M., Tugushev M., Prapas N., Bizzarri M., Espinola M. S. B., Di Lorenzo C., Ozay A. C., Nestler J., When one size does not fit all: Reconsidering PCOS etiology, diagnosis, clinical subgroups, and subgroup-specific treatments. Endocr. Metab. Sci. 14, 100159 (2024).
-
- Escobar-Morreale H. F., Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 14, 270–284 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
