Human and zebrafish mineralocorticoid receptors reporter cell assays to assess the activity of chemicals
- PMID: 41280684
- PMCID: PMC12636382
- DOI: 10.1016/j.isci.2025.113764
Human and zebrafish mineralocorticoid receptors reporter cell assays to assess the activity of chemicals
Abstract
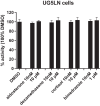
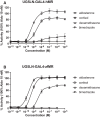
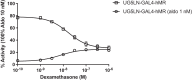
The action of environmental chemicals (ECs) on the mineralocorticoid receptor (MR) has been suggested to impair physiological processes regulated by this nuclear receptor. However, it remains understudied both as a target of ECs and with respect to potential species-specific differences. In this regard, we have developed reporter cell lines to identify the response to different steroids, ECs, and urban wastewater (WW) sample extracts of human MR (hMR) and zebrafish MR (zfMR). Most of the steroids had a higher efficacy on zfMR than hMR, while the ECs were antagonists to both hMR and zfMR, with a lower potency on the latter. Interestingly, WW sample extracts revealed the presence of MR activity with a greater activity on zfMR compared to hMR, suggesting the presence of steroids in WW. These screening tools have proven to be powerful tools for characterizing the interaction of chemicals with MRs and revealing their presence in environmental samples.
Keywords: Biochemistry; Biological sciences; Cell biology; Environmental monitoring; Natural sciences.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Amir S., Shah S.T.A., Mamoulakis C., Docea A.O., Kalantzi O.I., Zachariou A., Calina D., Carvalho F., Sofikitis N., Makrigiannakis A., Tsatsakis A. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public Health. 2021;18:1464. doi: 10.3390/ijerph18041464. - DOI - PMC - PubMed
-
- Delfosse V., Grimaldi M., Pons J.L., Boulahtouf A., le Maire A., Cavailles V., Labesse G., Bourguet W., Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA. 2012;109:14930–14935. doi: 10.1073/pnas.1203574109. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

