In-Depth Analysis of the Paramagnetic Properties in DHI/DHICA-Controlled Eumelanin
- PMID: 41280797
- PMCID: PMC12631427
- DOI: 10.1021/acsomega.5c08896
In-Depth Analysis of the Paramagnetic Properties in DHI/DHICA-Controlled Eumelanin
Abstract
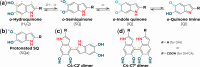
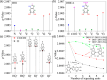
Eumelanin, a naturally occurring pigment derived from the oxidative polymerization of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA), has been extensively studied for its role in human biology and its potential applications in biomedical and sustainable electronic fields. By employing X-band electron paramagnetic resonance spectroscopy, this study focuses on eumelanin with different DHI/DHICA ratios, directly comparing monomeric and polymeric forms. The analysis reveals significant differences in the paramagnetic profiles, with changes in spin dynamics and relaxation times closely linked to the distinct macrostructures formed during polymerization. These findings offer valuable insights into how the molecular architecture of eumelanin influences its paramagnetic environments.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
-
- Paulin J. V., Graeff C. F. O.. From Nature to Organic (Bio)Electronics: A Review on a Melanin-Inspired Material. J. Mater. Chem. C. 2021;9:14514–14531. doi: 10.1039/D1TC03029A. - DOI
-
- Paulin J. V.. A Greener Prescription: The Power of Natural Organic Materials in Healthcare. RSC Sustain. 2024;2:2190–2198. doi: 10.1039/D4SU00219A. - DOI
-
- Piacenti-Silva M., Matos A. A., Paulin J. V., Alavarce R. A. da S., de Oliveira R. C., Graeff C. F.. Biocompatibility Investigations of Synthetic Melanin and Melanin Analogue for Application in Bioelectronics. Polym. Int. 2016;65:1347–1354. doi: 10.1002/pi.5192. - DOI
LinkOut - more resources
Full Text Sources
