Mesenchymal stem cells' exosomes alleviate chronic visceral pain through Nrf-2-mediated oxidative stress pathway in rats
- PMID: 41280947
- PMCID: PMC12634240
- DOI: 10.1097/PR9.0000000000001372
Mesenchymal stem cells' exosomes alleviate chronic visceral pain through Nrf-2-mediated oxidative stress pathway in rats
Abstract
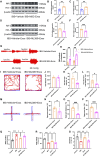
Introduction: Irritable bowel syndrome (IBS) is a prevalent chronic functional gastrointestinal disorder characterized by visceral hypersensitivity (VH), affecting over 10% of the global population. Current treatments for IBS have notable limitations, highlighting the need for novel therapeutic strategies to address chronic visceral pain and associated comorbidities. Objectives: This study aimed to investigate whether bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos) can alleviate chronic visceral pain in IBS and to explore the underlying molecular mechanisms, with a specific focus on the Nrf-2/HO-1 oxidative stress pathway.
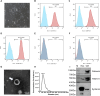
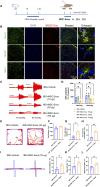
Methods: High-quality BMSC-Exos were isolated and characterized. In vitro, their neuroprotective effects were assessed in oxidatively stressed neurons. In vivo, IBS model rats received intrathecal injections of BMSC-Exos, and their effects on visceral pain sensitivity and anxiety-like behaviors were evaluated. Spinal cord tissues were analyzed to determine modulation of the Nrf-2/HO-1 pathway.
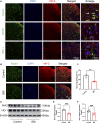
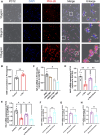
Results: In vitro studies demonstrated that BMSC-Exos effectively rescued oxidative stress-induced neuronal damage. In IBS rats, intrathecally administered BMSC-Exos were internalized by spinal cord neurons, significantly reducing visceral hypersensitivity and anxiety-like behaviors. Mechanistically, BMSC-Exos upregulated the Nrf-2/HO-1 antioxidant pathway, mitigating oxidative stress-induced neuronal damage.
Conclusion: These findings demonstrate that BMSC-Exos alleviate chronic visceral pain and comorbid anxiety in IBS rats, likely through Nrf-2/HO-1-mediated oxidative stress reduction in spinal neurons. These results highlight BMSC-Exos as a promising acellular therapeutic strategy for IBS, offering potential applications for this debilitating disorder.
Keywords: Anxiety mood; Bone marrow mesenchymal stem cell–derived exosomes; Chronic visceral hyperalgesia; Nrf-2/HO-1.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflict of interest to declare.Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Chen Y, Chen AQ, Luo XQ, Guo LX, Tang Y, Bao CJ, Lin L, Lin C. Hippocampal NR2B-containing NMDA receptors enhance long-term potentiation in rats with chronic visceral pain. Brain Res 2014;1570:43–53. - PubMed
-
- Chen Y, Feng S, Li Y, Zhang C, Chao G, Zhang S. Gut microbiota and intestinal immunity—a crosstalk in irritable bowel syndrome. Immunology 2024;172:1–20. - PubMed
-
- Evangelista AF, Vannier-Santos MA, de Assis Silva GS, Silva DN, Juiz PJL, Nonaka CKV, Dos Santos RR, Soares MBP, Villarreal CF. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation 2018;15:189. - PMC - PubMed
LinkOut - more resources
Full Text Sources
