A novel protocol for protoplast isolation, transfection, and culture in Cannabis sativa L
- PMID: 41286625
- PMCID: PMC12642208
- DOI: 10.1186/s12870-025-07686-1
A novel protocol for protoplast isolation, transfection, and culture in Cannabis sativa L
Abstract
Background: Protoplasts are a valuable tool for studying gene expression and applying genome editing techniques. Given the high medicinal and industrial potential of Cannabis sativa L., developing an efficient protoplast-to-plant regeneration protocol is highly desirable. Due to its recalcitrant nature, a complete plant regeneration from cannabis protoplasts has not yet been achieved.
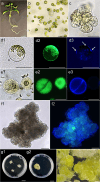
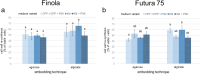
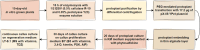
Results: This study details a robust protocol for cannabis protoplast isolation, purification, transient transfection, and culture, additionally reporting somatic embryo-like structures derived from protoplast-derived callus. We demonstrated that the age of donor material, the composition of the enzyme solution, and the duration of enzymolysis are crucial for efficient protoplast isolation. Protoplast embedding, coupled with a rich culture medium and plant growth regulators, proved critical for initiating cell wall re-synthesis, cell division, and microcallus formation. Protoplasts isolated using the reported protocol were abundant (2.2 × 106 protoplasts/1 g of fresh weight), viable (78.8% viability) and able to undergo cell wall re-synthesis (56.1% of viable cells), followed by cell divisions (15.8% plating efficiency). Polyethylene glycol-mediated transfection yielded a 28% transfection efficiency and 17% plating efficiency in 10-day cultures. Protoplast-derived microcalli successfully proliferated on six regeneration media containing various concentrations of 6-benzylaminopurine and thidiazuron, exhibiting further proliferation and greening within two months.
Conclusions: This system provides a reliable protocol for isolation, transfection and culture of cannabis protoplasts. It also offers a framework for investigating gene function, as well as advancing protoplast fusion and genome editing technologies for this species.
Keywords: 2-aminoindane-2-phosphonic acid; Hemp; Phytosulfokine; Protoplast embedding; Tissue cultures.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Grand View Research market analysis report. Industrial hemp market size, share & trends analysis report by application (animal care, textiles, food & beverages), by product (seeds, fiber, shivs), by region (North America, Asia Pacific), and segment forecasts, 2024–2030. www.grandviewresearch.com/industry-analysis/industrial-hemp-market. Assessed 23 July 2025.
-
- Andersson M, Turesson H, Olsson N, Fält A-S, Ohlsson P, Gonzalez MN, et al. Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol Plant. 2018;164:378–84. - PubMed
-
- Park J, Choi S, Park S, Yoon J, Park AY, Choe S. DNA-Free genome editing via ribonucleoprotein (RNP) delivery of CRISPR/Cas in lettuce. In: Qi Y, editor. Plant genome editing with CRISPR systems: methods and protocols. New York, NY: Springer; 2019. pp. 337–54. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

