Exploring a bimetallic catalyst family for hydrogen oxidation with insights into superior activity and durability
- PMID: 41290687
- PMCID: PMC12647760
- DOI: 10.1038/s41467-025-65503-7
Exploring a bimetallic catalyst family for hydrogen oxidation with insights into superior activity and durability
Abstract
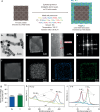
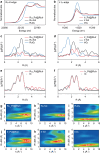
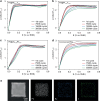
Anion exchange membrane fuel cells are limited by the slow kinetics of the alkaline hydrogen oxidation reaction (HOR). Aided by density functional theory combined with fine-tuned machine learning interatomic potential, we establish a family of bimetallic catalysts with controlled surface atomic arrangements to identify the optimal catalysts for HOR. Our theoretical analysis successfully predicts the HOR activity rankings of these catalysts, consistent with the experimental results. RuIr exhibits the highest activity, followed by PtRu, AuIr, PtRh, PtIr, PtAu, RhIr, RuRh, AuRu, and AuRh. These trends correlate with the electron-accepting tendencies and the adsorption strengths of H2 and OH* on the catalysts. Among all candidates, RuIr emerges as the most active and durable bimetallic catalyst. Furthermore, operando X-ray absorption spectroscopy and electrochemical measurements reveal a strong synergistic effect of RuIr, where Ir exhibits superior electron-accepting tendency and strong H2 adsorption, while Ru demonstrates strong OH* adsorption, accelerating the alkaline HOR process.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci.7, 2255–2260 (2014).
-
- Sheng, W., Gasteiger, H. A. & Shao, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc.157, B1529 (2010).
-
- Sheng, W. et al. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun.6, 1–6 (2015). - PubMed
-
- Cong, Y., Yi, B. & Song, Y. Hydrogen oxidation reaction in alkaline media: from mechanism to recent electrocatalysts. Nano Energy44, 288–303 (2018).
-
- Mu, X., Liu, S., Chen, L. & Mu, S. Alkaline hydrogen oxidation reaction catalysts: insight into catalytic mechanisms, classification, activity regulation and challenges. Small Struct.4, 2200281 (2023).
LinkOut - more resources
Full Text Sources

