JMT103 versus Non-Denosumab or Denosumab Treatment in Chinese Patients with Unresectable or Surgically Challenging Giant Cell Tumor of Bone: A Propensity Score-Matched Comparison
- PMID: 41292259
- PMCID: PMC12647920
- DOI: 10.1002/cam4.71340
JMT103 versus Non-Denosumab or Denosumab Treatment in Chinese Patients with Unresectable or Surgically Challenging Giant Cell Tumor of Bone: A Propensity Score-Matched Comparison
Abstract
Background: Giant cell tumors of bone (GCTB) are RANK/RANK-ligand positive, progressive osteolytic tumors. There was no medical treatment for GCTB based on efficacy and safety data from Chinese patients. A single-arm, phase II study demonstrated the promising efficacy of JMT103 in unresectable or surgically challenging GCTB. Patient-level outcomes from the single-arm trial were compared with real-world data from denosumab or non-denosumab treated patients to estimate comparative efficacy in unresectable or surgically challenging GCTB.
Methods: The real-world data were retrospectively collected from three hospitals between January 2013 and December 2023. The eligibility criteria of the two cohorts were based on the JMT103 single-arm study. Propensity score matching was used to balance the baseline characteristics of patients in the JMT103 and the real-world cohorts. The primary endpoint was histopathological or 12 week radiological objective tumor response rate (OTR). Secondary endpoints included tumor response rate throughout the study, objective response rate, disease control rate, and safety profiles.
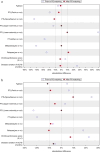
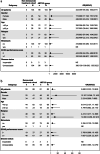
Results: 166 and 135 patients were finally included in the non-denosumab and denosumab cohorts, respectively. After 1:1 nearest neighbor matching, the OTR of the JMT103 cohort was significantly higher than that of the non-denosumab cohort (94.2% vs. 4.8%), and was comparable with that of the denosumab cohort (92.0% vs. 67.0%). The same results were observed in tumor response rate throughout the study (JMT103 vs. non-denosumab: 94.2% vs. 4.8%, JMT103 vs. denosumab: 94.3% vs. 86.4%), objective response rate (83.5% vs. 11.1%, 87.4% vs. 80.0%), and disease control rate (100% vs. 70.4%, 100.0% vs. 98.8%).
Conclusion: JMT103 has manageable safety profiles with better effectiveness than non-denosumab and a trend toward greater effectiveness than denosumab in patients with unresectable or surgically challenging GCTB.
Trial registration: NCT05402865, NCT04255576.
Keywords: JMT103; effectiveness; giant cell tumor of bone; propensity score analysis; safety.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The results were presented in part as an oral presentation at the annual meeting of the Chinese Society of Clinical Oncology (CSCO) 2022.
J.W. and H.L. are employees of CSPC Pharmaceutical Group Limited. The other authors have stated that they have no conflicts of interest.
Figures

References
-
- Verschoor A. J., Bovée J., Mastboom M. J. L., Sander Dijkstra P. D., Van De Sande M. A. J., and Gelderblom H., “Incidence and Demographics of Giant Cell Tumor of Bone in The Netherlands: First Nationwide Pathology Registry Study,” Acta Orthopaedica 89, no. 5 (2018): 570–574, 10.1080/17453674.2018.1490987. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

