Exceptional Response to Trastuzumab Deruxtecan (T-DXd) in HER2-Positive Metastatic Endometrial Cancer
- PMID: 41294658
- PMCID: PMC12651274
- DOI: 10.3390/curroncol32110596
Exceptional Response to Trastuzumab Deruxtecan (T-DXd) in HER2-Positive Metastatic Endometrial Cancer
Abstract
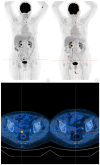
Objectives: Endometrial cancer is the most common gynaecologic malignancy, and its mortality rate is rising. Advanced or recurrent disease remains challenging because historically there have been limited therapeutic options. We aim to describe a complete and durable response to the HER2-directed antibody-drug conjugate trastuzumab deruxtecan (T-DXd) in a heavily pretreated patient with HER2-positive, mismatch-repair-deficient metastatic serous endometrial cancer. Methods: A 72-year-old woman underwent hysterectomy, bilateral salpingo-oophorectomy, and staging procedures for FIGO stage IIIA, high-grade serous papillary endometrial carcinoma. Tumour profiling revealed dMMR, a p53 abnormal pattern, and HER2 overexpression (IHC 3+). She received carboplatin/paclitaxel plus avelumab, followed by pegylated liposomal doxorubicin and weekly paclitaxel. After progression on paclitaxel, off-label T-DXd was initiated. Molecular data (FoundationOne CDx) were collected, along with and serial imaging and CA125 assessments. Results: The patient developed cough after two cycles of T-DXd; interstitial lung disease was excluded, and treatment resumed with steroid cover. By December 2024, PET/CT demonstrated complete metabolic response, with resolution of vaginal-vault and para-aortic lesions and normalisation of CA125. Real-world progression-free survival exceeded eight months, with ongoing symptom improvement. Treatment was generally well tolerated; the principal adverse event was grade 3 neutropenia requiring dose reduction. No cardiotoxicity or interstitial lung disease occurred. Conclusions: This case illustrates that T-DXd can induce deep and durable remission in HER2-positive, dMMR metastatic serous endometrial cancer after multiple lines of therapy. It adds real-world evidence supporting further investigation of HER2-directed antibody-drug conjugates in gynaecologic malignancies, and underscores the need for confirmatory trials and refined biomarker-driven patient selection.
Keywords: endometrial cancer; targeted therapy.
Conflict of interest statement
F.P. reported honoraria for advisory boards, activities as a speaker, travel grants, and research grants from AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Exact Sciences, Gilead, Italfarmaco, Menarini, MSD, Novartis, Pierre Fabre, Pfizer, and Roche outside the submitted work. MB reported honoraria for advisory boards and speaker fees for GSK, Eisai, AstraZeneca, MSD, Roche, and AbbVie outside the submitted work.
Figures
References
-
- Monk B.J., Smith G., Lima J., Long G.H., Alam N., Nakamura H., Meulendijks D., Ghiorghiu D., Banerjee S. Real-world outcomes in patients with advanced endometrial cancer: A retrospective cohort study of US electronic health records. Gynecol. Oncol. 2022;164:325–332. doi: 10.1016/j.ygyno.2021.12.008. - DOI - PubMed
-
- Bartoletti M., Montico M., Lorusso D., Mazzeo R., Oaknin A., Musacchio L., Scambia G., Puglisi F., Pignata S. Incorporation of anti-PD1 or anti PD-L1 agents to platinum-based chemotherapy for the primary treatment of advanced or recurrent endometrial cancer. A meta-analysis. Cancer Treat. Rev. 2024;125:102701. doi: 10.1016/j.ctrv.2024.102701. - DOI - PubMed
-
- Pignata S., Scambia G., Schettino C., Arenare L., Pisano C., Lombardi D., De Giorgi U., Andreetta C., Cinieri S., De Angelis C., et al. Carboplatin and paclitaxel plus avelumab compared with carboplatin and paclitaxel in advanced or recurrent endometrial cancer (MITO END-3): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2023;24:286–296. doi: 10.1016/S1470-2045(23)00016-5. - DOI - PubMed
-
- Pignata S., Califano D., Lorusso D., Arenare L., Bartoletti M., De Giorgi U., Andreetta C., Pisano C., Scambia G., Lombardi D., et al. MITO END-3: Efficacy of avelumab immunotherapy according to molecular profiling in first-line endometrial cancer therapy. Ann. Oncol. 2024;35:667–676. doi: 10.1016/j.annonc.2024.04.007. - DOI - PubMed
-
- Meric-Bernstam F., Makker V., Oaknin A., Oh D.-Y., Banerjee S., González-Martín A., Jung K.H., Ługowska I., Manso L., Manzano A., et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024;42:47–58. doi: 10.1200/JCO.23.02005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous