S1P3 Receptor Mediates the Proinflammatory Effect of the Endocannabinoid 2-Arachidonoylglycerol in Endometriotic Epithelial Cells
- PMID: 41294926
- PMCID: PMC12651104
- DOI: 10.1096/fj.202502415R
S1P3 Receptor Mediates the Proinflammatory Effect of the Endocannabinoid 2-Arachidonoylglycerol in Endometriotic Epithelial Cells
Abstract
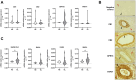
Endometriosis is a chronic inflammatory disease characterized by the ectopic implantation of endometrium outside the uterus associated with pelvic pain and infertility. The molecular mechanisms involved in the pathogenesis of endometriosis are complex and far from being fully elucidated. We recently showed that the signaling of the bioactive sphingolipid sphingosine 1-phosphate (S1P) is deeply dysregulated in endometriosis. The endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), via ligation to G-protein coupled receptors, CB1, CB2, and GPR18 as well as the cation channel TRPV1, play a crucial role in the modulation of pain and inflammation. Here, the role of endocannabinoid signaling in endometriosis and its possible cross talk with the S1P signaling axis has been investigated. It has been found that CB1, CB2, GPR18, TRPV1 as well as the enzymes involved in endocannabinoid metabolism are expressed in endometriotic lesions. Furthermore, the effect of 2-AG and AEA in the modulation of inflammation has been established in human endometriotic epithelial cells. 2-AG, but not methanandamide (MAEA), the nonhydrolyzable AEA analogue, induced a marked increase in the expression of cyclooxygenase 2 and various pro-inflammatory interleukins (IL-1β, IL-6 and IL-8). Interestingly, S1P3, whose expression is augmented by 2-AG, is crucial for transducing the biological action of the endocannabinoid. Indeed, S1P3 pharmacological blockade or its specific silencing impaired the pro-inflammatory action of 2-AG. In conclusion, these findings demonstrate, for the first time, the occurrence of a functional interplay between endocannabinoids and S1P signaling in endometriosis, paving the way for novel pharmacological strategies to treat the disease.
Keywords: 2‐arachidonoylglycerol; S1P receptors; endocannabinoid system; endometriosis; inflammation; sphingosine 1‐phosphate.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Joshi N. and Onaivi E. S., “Endocannabinoid System Components: Overview and Tissue Distribution,” in Recent Advances in Cannabinoid Physiology and Pathology, vol. 1162, ed. Bukiya A. N. (Springer International Publishing, 2019), 1–12. - PubMed
-
- Gatta‐Cherifi B. and Cota D., “New Insights on the Role of the Endocannabinoid System in the Regulation of Energy Balance,” International Journal of Obesity 40 (2016): 210–219. - PubMed
