Maternal and Fetal SERPINA3 Polymorphisms and Risk of Preeclampsia: A Dyad and Triad Based Case-Control Study
- PMID: 41296456
- PMCID: PMC12651244
- DOI: 10.3390/cimb47110952
Maternal and Fetal SERPINA3 Polymorphisms and Risk of Preeclampsia: A Dyad and Triad Based Case-Control Study
Abstract
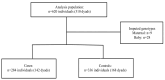
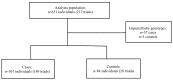
Serine protease inhibitor A3 (SERPINA3), also called α-1-antichymotrypsin, is a serine protease involved in placental dysfunction. This study examines SERPINA3 polymorphisms and haplotypes for associations with maternal hypertensive disorders of pregnancy (HDPs) and preeclampsia with severe features (sPE) or Hemolysis, Elevated Liver Enzymes, and Low Platelet (HELLP) syndrome in mother-baby dyads (HDP) and mother-father-baby triads (sPE/HELLP). This retrospective case-control study examined two patient cohorts, HDPs and severe PE/HELLP syndrome. The HDP population included cases (n = 142) and controls (n = 168) of mother-baby dyads recruited from a large, urban, safety-net hospital in Los Angeles. The sPE/HELLP syndrome population included cases (n = 189) and controls (n = 28) of mother-father-baby triads recruited through HELLP syndrome research websites. Cases were verified by medical chart abstraction when possible. Two SERPINA3 SNPs, rs4934 and rs1884082, were genotyped from saliva samples, mouthwash, or buccal swabs. The Haplin package in R was used to perform genetic association analyses. No evidence of increased risk related to individual SERPINA3 SNPs or haplotypes for the developing HDPs or sPE/HELLP was found in individual nor combined cohorts. In the HDP cohort, the g-a haplotype (relative to T-G haplotype) was borderline significant for increased risk of HDPs when carried by the child (double dose: RR = 1.58, 95% CI: (1.00, 2.52), p = 0.05). We observed significant parent-of-origin (PoO) effects in the combined cohort: specifically, an increased risk of HDPs/sPE/HELLP if the mother carries a double copy for both rs4934 (RR = 3.03, 95% CI (1.50, 6.09), p < 0.01) and rs1884082 (RR = 2.38, 95% CI (1.22, 4.71), p = 0.01). A reduced risk of HDPs/sPE/HELLP was observed for rs4934 (RR = 0.54, 95% CI (0.31, 0.98), p = 0.04) and rs1884082 (RR = 0.52, 95% CI (0.30, 0.91), p = 0.02) with child carriage of the maternally inherited allele. In contrast, child carriage of a paternally inherited copy of the variant allele for rs4934 increased risk of HDPs/sPE/HELLP (RR = 1.54, 95% CI (1.09, 2.20), p = 0.02). There was no evidence that SERPINA3 gene polymorphisms and haplotypes were associated with risk of HDPs or sPE/HELLP. However, significant PoO effects were observed in the combined cohort analysis, with child carriage of rs4934 that is maternally inherited decreasing HDPs/sPE/HELLP risk while a paternally inherited copy increases risk, suggesting a role for maternal-fetal genomic incompatibility.
Keywords: HELLP syndrome; genetics; parent-of-origin; polymorphism; preeclampsia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- US Preventive Services Task Force. Barry M.J., Nicholson W.K., Silverstein M., Cabana M.D., Chelmow D., Coker T.R., Davis E.M., Donahue K.E., Jaén C.R., et al. Screening for Hypertensive Disorders of Pregnancy: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2023;330:1074–1082. doi: 10.1001/jama.2023.16991. - DOI - PubMed
-
- Mathew R., Devanesan B.P., Sreedevi N. Prevalence of hypertensive disorders of pregnancy, associated factors, and pregnancy complications in a primigravida population. Gynecol. Obstet. Clin. Med. 2023;3:119–123. doi: 10.1016/j.gocm.2023.01.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous