Interplay among lipoprotein(a), hepatic and vascular damage in individuals with metabolic dysfunction
- PMID: 41299661
- PMCID: PMC12659110
- DOI: 10.1186/s12933-025-03004-z
Interplay among lipoprotein(a), hepatic and vascular damage in individuals with metabolic dysfunction
Abstract
Background: The relationship between plasma lipoprotein(a) [Lp(a)] levels and metabolic dysfunction-associated steatotic liver disease (MASLD) remains unclear. The aim of this study was to examine the combined effects of Lp(a) levels on liver and vascular damage.
Methods: The study was conducted using the Liver-Bible cohort of individuals with metabolic dysfunction (n = 859, 808 with genomic information) and the Milan Biobank (n = 6963). Genome-wide association studies (GWAS) and polygenic risk scores (PRS) were used to evaluate the inherited factors influencing plasma Lp(a) levels.
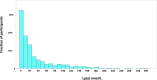
Results: In the Liver-Bible cohort, genetic variation in the LPA gene was the strongest determinant of Lp(a), followed by liver stiffness measurement (LSM). Additionally, circulating Lp(a) levels, but not genetic predisposition, were inversely related to LSM, suggesting that MASLD severity may affect Lp(a) secretion. Among participants with more severe insulin resistance (n = 250), Lp(a) levels (odds ratio 6.7, 95% CI 1.0-53.0, p = 0.046) and LSM (odds ratio 13.7, 95% CI 1.4-172.2, p = 0.023) were associated with greater prevalence of carotid atherosclerotic plaques, regardless of traditional cardiovascular risk factors. In the Milan Biobank, genetically predicted higher Lp(a) levels tended to increase the risk of liver-related outcomes, whereas genetically predicted MASLD was associated with lower circulating Lp(a) levels.
Conclusions: The results of this study suggest that liver damage is more likely the cause of reduced plasma Lp(a) levels rather than a consequence. Assessing plasma Lp(a) levels and the extent of liver damage could improve the prediction of vascular damage.
Keywords: Atherosclerosis; Fibrosis; Lipoprotein; Liver stiffness measurement; MASLD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethical Committee of the Fondazione IRCCS Ca’ Granda, and each participant signed a written informed consent (ID 1650, revision June 23rd, 2020). Consent for publication: N/A, see ethical consideration. Competing interests: None of the authors report any conflicts of interest relevant for the present manuscript. LV reports speaking fees from: Viatris, Novo Nordisk, GSK; consulting for: Novo Nordisk, Pfizer, Boehringer Ingelheim, Resalis, Almac. JMM reports speaking fees and consulting from Abbott.
Figures
References
-
- Mora S, Kronenberg F. Lipoprotein(a). JAMA. 2025;333:1918–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

