Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance
- PMID: 41300192
- PMCID: PMC12650465
- DOI: 10.3390/brainsci15111185
Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance
Abstract
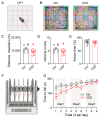
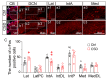
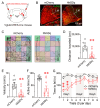
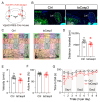
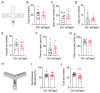
Background/Objectives: The cerebellum is essential for motor coordination and has recently been implicated in sleep-related disorders. However, the neural mechanisms linking sleep disruption to motor dysfunction remain poorly understood. This study aimed to elucidate the roles of the deep cerebellar nuclei (DCN), particularly the lateral nucleus, in motor dysfunction induced by chronic sleep disruption (CSD). Methods: Using a validated mouse model of CSD with periodic sleep fragmentation induced by an orbital shaker during the light phase, we assessed neuronal activation via c-Fos immunostaining and performed chemogenetic manipulation of glutamatergic neurons within the lateral nucleus. Behavioral performance was evaluated using open-field and rotarod tests. Results: CSD selectively increased c-Fos expression in the lateral nucleus, with no significant changes observed in other DCN subregions. Chemogenetic activation or ablation of glutamatergic neurons in the lateral nucleus decreased locomotor activity in the open-field test and shortened latency to fall in the rotarod task. Conversely, chemogenetic inhibition of these neurons attenuated CSD-induced impairments, restoring locomotor performance toward control levels. Conclusions: Our findings provide direct experimental evidence that glutamatergic neurons in the lateral nucleus play a crucial role in mediating CSD-induced motor dysfunction. These results highlight the cerebellar contribution to the interplay between sleep and motor control and identify a potential target for therapeutic intervention in sleep-related motor disorders.
Keywords: cerebellum; chronic sleep disruption; deep cerebellar nuclei; glutamatergic neurons; lateral nucleus; motor dysfunction.
Conflict of interest statement
All other authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

