Novel Chalcone Derivatives as Anti- Leishmania infantum Agents with Potential Synergistic Activity and In Silico Insights
- PMID: 41301618
- PMCID: PMC12649483
- DOI: 10.3390/antibiotics14111123
Novel Chalcone Derivatives as Anti- Leishmania infantum Agents with Potential Synergistic Activity and In Silico Insights
Abstract
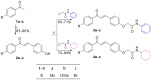
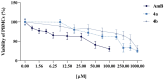
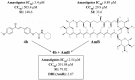
Background: Visceral leishmaniasis (VL) is a neglected tropical disease with limited therapeutic options, often restricted by toxicity, high costs, and resistance. Chalcones are promising scaffolds for the development of antiparasitic agents. Objectives: This study aimed to synthesize novel acetamides derived from 4-hydroxychalcones and evaluate their antileishmanial activity, cytotoxicity, potential synergy with amphotericin B (AmB), and mechanisms of action through in silico analyses. Methods: Six chalcone-acetamides (3a-c, 4a-c) were synthesized and characterized by IR, NMR, and HRMS. In vitro activity against Leishmania infantum promastigotes and axenic amastigotes was assessed by colorimetric assays. Cytotoxicity was tested in human erythrocytes and PBMCs. Synergy with AmB was analyzed by the combination index. Molecular docking targeted parasite enzymes, and ADMET tools predicted pharmacokinetic and safety profiles. Results: Phenyl-substituted derivatives (3a-c) were inactive, while cyclohexyl-substituted analogs (4a-c) were active. Compound 4b displayed the strongest effect (IC50: 7.02 μM for promastigotes, 3.4 μM for amastigotes), with low cytotoxicity and high Selectivity Indices. In combination with AmB, compound 4b reduced the effective dose (DRI: 2.87) and increased the therapeutic window. Docking revealed favorable interactions of compound 4b with deubiquitinase DUB16 and tryparedoxin peroxidase I, suggesting enzyme inhibition. ADMET predictions supported good absorption and low toxicity. Conclusions: Compound 4b demonstrated potent and selective antileishmanial activity, synergism with AmB, and predicted safety. These findings highlight chalcone derivative 4b as a promising lead for future preclinical development in VL therapy.
Keywords: acetamides; antileishmanial; flavonoids; synergy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Yasmin H., Adhikary A., Al-Ahdal M.N., Roy S., Kishore U. Host–pathogen interaction in leishmaniasis: Immune response and vaccination strategies. Immuno. 2022;2:218–254. doi: 10.3390/immuno2010015. - DOI
-
- Pan-American Health Organization (PAHO) Leishmaniasis: Epidemiological Report of the Americas Region. 2024. [(accessed on 3 November 2025)]. Available online: https://iris.paho.org/handle/10665.2/51742.
Grants and funding
LinkOut - more resources
Full Text Sources

