A Deadly Duet: Fanconi Anemia (FA) With Head and Neck Cancer
- PMID: 41306138
- PMCID: PMC12646129
- DOI: 10.7759/cureus.95448
A Deadly Duet: Fanconi Anemia (FA) With Head and Neck Cancer
Abstract
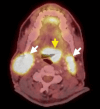
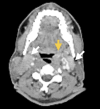
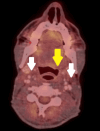
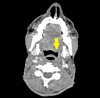
Fanconi anemia (FA) is a rare, inherited disorder characterized by chromosomal instability, bone marrow failure, and predisposition to malignancies, including head and neck squamous cell carcinoma (HNSCC). The treatment of HNSCC in FA is challenging due to the extreme sensitivity of these patients to DNA-damaging agents. A 39-year-old male with FA presented with odynophagia and neck swelling. Examination revealed a mass at the base of the tongue (BOT) with bilateral cervical lymphadenopathy. Histopathology confirmed moderately differentiated squamous cell carcinoma (MD-SCC) (p16 negative). He underwent induction chemotherapy but developed severe myelosuppression. In view of the unusual clinical presentation, a mitomycin stress test was performed, confirming FA. Consequently, further chemotherapy was deferred, and radiotherapy was delivered with careful monitoring and supportive care. The patient achieved a complete metabolic response post-treatment. The case highlights the importance of radiation treatment in FA patients with exaggerated chemotherapy toxicities and the need for tailored management in this patient population.
Keywords: chemotherapy-induced myelosuppression; conventional radiotherapy; fanconi anemia; head and neck cancer; inherited disorder.
Copyright © 2025, D et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Fanconi Anemia Research Fund. Fanconi Anemia Clinical Care Guidelines, 5th ed. Eugene. Eugene, USA: Fanconi Cancer Foundation; 2020.
-
- Cancer incidence in persons with Fanconi anemia. Rosenberg PS, Greene MH, Alter BP. Blood. 2003;101:822–826. - PubMed
-
- High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Kutler DI, Auerbach AD, Satagopan J, et al. Arch Otolaryngol Head Neck Surg. 2003;129:106–112. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
