Soluble suppression of tumorigenicity-2 changes during cardiotoxic cancer treatment: a systematic review and meta-analysis
- PMID: 41306265
- PMCID: PMC12645410
- DOI: 10.3389/fcvm.2025.1624023
Soluble suppression of tumorigenicity-2 changes during cardiotoxic cancer treatment: a systematic review and meta-analysis
Abstract
Background: Soluble suppression of tumorigenicity-2 (sST2) is a promising biomarker of cardiovascular disease and heart failure. Data about the changes in sST2 concentrations during cancer treatment and the relationship with cancer treatment-related cardiotoxicity are sparse.
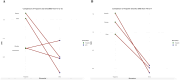
Methods: We conducted a systematic review and meta-analysis to explore longitudinal changes in sST2 levels at three time points (T0 baseline, T1 post-chemotherapy, and T2 follow-up) in cancer patients treated with cardiotoxic therapies and compared these changes to traditional biomarkers of cardiac injury, i.e., troponin and NT-proBNP. Using random-effects models, mean differences (MD), and standardized MD (SMD), we analyzed (i) ST2 longitudinal changes, (ii) the association between ST2 and cardiotoxicity [defined through left ventricular ejection fraction (LVEF)] providing pooled estimates of correlations, and (iii) the SMD variations among biomarkers.
Results: Eight studies were included, comprising 433 patients treated with anthracycline and/or HER2-directed antibodies. There was a trend toward increased sST2 levels from T0 to T2 (MD 1.86, 95% CI -0.97 to 4.68, p = 0.200) and decreased levels from T1 to T2 (MD -1.96, 95% CI -4.28 to 0.37, p = 0.100). A pooled analysis showed a negative correlation between sST2 levels and LVEF (r -0.29, 95% CI, -0.49- -0.05, p < 0.010). Comparisons with Troponin and NT-proBNP showed a significantly higher Troponin SMD at T0-T1 (p = 0.027), while no significant differences were observed for NT-proBNP.
Conclusion: sST2 showed dynamic changes during cardiotoxic therapy correlating with cardiotoxicity. Troponin was demonstrated to have greater longitudinal variations. Further research is needed to evaluate longitudinal sST2 levels in patients who develop cardiotoxicity vs. those who do not.
Keywords: ST2; biomarker; cardioncology; cardiotoxcity; cardiotoxic adverse effect.
© 2025 Fazzini, Angius, Campana, Pascalis, Deidda, Pugliesi, Quagliariello, Maurea, Tocchetti, Ameri and Cadeddu Dessalvi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the cardio-oncology study group of the heart failure association of the European society of cardiology in collaboration with the international cardio-oncology society. Eur J Heart Fail. (2020) 22(11):1945–60. 10.1002/ejhf.1920 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

