Efficacy and Safety of PRaG Therapy in Elderly Patients with Advanced Malignant Tumors: A Prospective, Multicenter Clinical Study Protocol (PRaG 9.0 Study)
- PMID: 41308040
- PMCID: PMC12660646
- DOI: 10.1177/15330338251400412
Efficacy and Safety of PRaG Therapy in Elderly Patients with Advanced Malignant Tumors: A Prospective, Multicenter Clinical Study Protocol (PRaG 9.0 Study)
Abstract
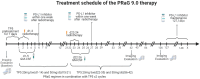
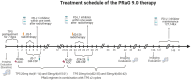
Background: Current evidence from evidence-based medicine is limited regarding the efficacy and safety of immunotherapy in elderly patients aged 75 years and older with malignant solid tumors. PRaG therapy, which combines PD-1/PD-L1 inhibitors, radiotherapy, and granulocyte-macrophage colony-stimulating factor (GM-CSF), aims to treat patients with advanced, refractory tumors. Preliminary findings indicate that patients aged 75 years and older can benefit from this treatment and can tolerate it well. Objective: This study aims to evaluate the efficacy and safety of the PRaG regimen in elderly patients with advanced malignant solid tumors to provide evidence-based support for immunotherapy in this population. Methods and Analysis: This study involves a multicenter, prospective, single-arm phase II clinical trial designed to enroll 29 patients aged 75 years and older with either newly diagnosed or recurrent metastatic advanced solid tumors that are histologically confirmed. All of the eligible patients will have had to receive at least two cycles of PRaG therapy until disease progression or intolerable adverse effects occurred. The study protocol was approved on September 12, 2023, by the Ethics Committee of the Second Affiliated Hospital of Soochow University (JD-LK-2023-082-I01) and by the ethics committees of all of the participating centers (Trial Registration Number: NCT06112041).
Keywords: GM-CSF; elderly cancer patients; immunotherapy; radiation therapy; the PRaG therapy; thymopentin.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials