Detection of spontaneous anti-neoepitope T-cell responses in non-metastatic bladder cancer patients
- PMID: 41312368
- PMCID: PMC12648094
- DOI: 10.3389/fimmu.2025.1627914
Detection of spontaneous anti-neoepitope T-cell responses in non-metastatic bladder cancer patients
Abstract
Background: Bladder carcinomas are immunogenic, and patients with bladder cancer benefit from immune checkpoint therapy. This is correlated to a high tumor mutation burden, which provides a higher number of neoepitopes that can be recognized by tumor-specific CD8+ T cells. Intravesical Bacillus Calmette-Guérin (BCG) is used to treat non-muscle invasive bladder cancer (NMIBC), but its mechanism of action remains elusive. Most lymphocytes appearing in the urine of BCG-treated patients are CD4+ T cells though preclinical studies showed that CD8+ T cells are also necessary for BCG treatment efficacy. It is currently unknown which proportion of patients with non-metastatic bladder cancer develop a spontaneous antitumor CD8+ response, and if BCG treatment influences this response.
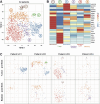
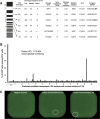
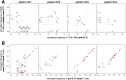
Methods: In a first cohort of 15 NMIBC and 9 muscle invasive bladder cancer patients, we used IFN-y ELISPOT assays to screen for the presence of anti-neoepitope CD8+ T cells in the blood, tumor and urine. In a second cohort of 4 NMIBC patients, we analyzed the features and specificity of CD8+ T cells infiltrating the tumoral or bladder tissues before and after BCG using single cell transcriptomic analyses. A total of 31 tumor-infiltrating CD8+ clonotypes were screened against neoepitopes and tumor cDNA libraries.
Results: 9 out of 24 patients from the first cohort mounted a spontaneous and functional anti-neoepitope T-cell response in blood and/or tumor. In 5 patients from this cohort who were treated with BCG, no neoantigen-specific T cells were detected in urine during treatment. In the second cohort, 6 out of 6 TCRs from exhausted CD8+ TILs from one patient recognized 5 different neoepitopes. T-cell receptor (TCR) repertoire analyses indicated that the frequencies of these tumor-specific T cells did not increase after BCG instillations, neither in the bladder nor in the blood. None of the 25 other TCRs of CD8+ T cells recognized tumor-specific antigens.
Conclusions: We show that one third of patients with non-metastatic bladder cancer mount a spontaneous and functional anti-neoepitope CD8+ T-cell response detectable in blood or tumor. In 4 patients with NMIBC, BCG treatment did not boost or induce the anti-neoepitope response, suggesting alternative mechanisms of action for its efficacy.
Keywords: BCG treatment; T lymphocyte; bladder cancer; immunotherapy; neoepitope.
Copyright © 2025 Brochier, Nguyen, Cesson, Bricard, van Baren, Hames, Dauguet, Dano, Tombal, Rodrigues-Dias, Masnada, Genolet, Harari, Roth, Lucca, Nardelli-Haefliger, Coulie and Derré.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. (2002) 168:1964–70. doi: 10.1016/S0022-5347(05)64273-5, PMID: - DOI - PubMed
-
- Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguie M, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. (2021) 22:919–30. doi: 10.1016/S1470-2045(21)00147-9, PMID: - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

