Sibiriline, a novel dual inhibitor of necroptosis and ferroptosis, prevents RIPK1 kinase activity and (phospho)lipid peroxidation as a potential therapeutic strategy
- PMID: 41315180
- PMCID: PMC12663298
- DOI: 10.1038/s41420-025-02852-8
Sibiriline, a novel dual inhibitor of necroptosis and ferroptosis, prevents RIPK1 kinase activity and (phospho)lipid peroxidation as a potential therapeutic strategy
Abstract
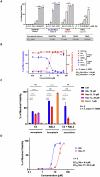
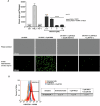
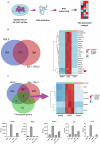
In the past two decades, various non-apoptotic pathways of regulated cell death have been identified; a small subset of these, including necroptosis and ferroptosis, manifests the phenotypic features of necrotic death. These two regulated necroses are being extensively studied because of their putative roles in severe acute and chronic pathologies. Moreover, as these regulated necrotic pathways are coactivated in a number of common pathologies, the development of multi-target directed ligands (that is, the use of a polypharmacological strategy) is a path-breaking avenue of research. In this study, we determined that the 7-azaindole derivative, sibiriline, inhibited both RIPK1-driven necroptosis (induced by Tumor Necrosis Factor-α) and ferroptosis (triggered by various classes of ferroptosis inducers), with EC50s against each in the µM range. We next performed a combined large-scale transcriptomic study in order to determine the molecular mechanisms of action of sibiriline. We identified the stress response protein heme oxygenase-1 (HMOX1) as the main biomarker of ferroptosis inhibition by sibiriline. We hypothesized that this compound reacts as an antioxidant to block ferroptosis; indeed, we found that sibiriline inhibits lipid peroxidation by trapping phospholipid-derived peroxyl radicals as a radical-trapping antioxidant (RTA). Taken together, these results show that sibiriline is a new dual inhibitor of necroptosis and ferroptosis cell death pathways; it works by inhibition of both RIPK1 kinase and (phospho)lipid peroxidation. We also demonstrate the in vitro efficacy of sibiriline to inhibit cell death in cell-based models of Parkinson's disease and cystic fibrosis. These findings shed light on the high therapeutic potency of RIPK1 inhibitors with RTA activity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Claire Delehouzé, Marie-Thérèse Dimanche-Boitrel, Morgane Rousselot and Stéphane Bach are the cofounders, shareholders and members of the scientific advisory board of SeaBeLife Biotech, which is developing novel therapies for treating liver acute disorders and retinal diseases. Peter Goekjian is member of the scientific advisory board of SeaBeLife Biotech. The other authors declare that they have no competing interests. Ethics approval and consent to participate: Patient data and tissue collection were performed under the agreement with the European Network of Research Ethics Committees and French ethic law. The ethical committee, according to the Medical Research Involving Human Subjects Act, reviewed and approved the study. Informed voluntary consent was obtained from every participant of the study: agreements CHU 19 244 C and CNRS 205782. Regarding consent, all patients are INFORMED about the use of their cells for research purposes. Patients could withdraw their consent at any time, leading to the prompt disposal of their tissue and any derived material.
Figures
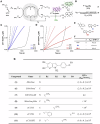
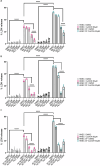
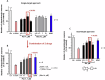
References
-
- Delanghe T, Dondelinger Y, Bertrand MJM. RIPK1 Kinase-dependent death: a symphony of phosphorylation events. Trends cell Biol. 2020;30:189–200. - PubMed
Grants and funding
- RGPIN-2022-05058/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- RGPIN-2022-05058/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- RGPIN-2022-05058/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- PhD CIFRE Grant 2019/0686/Agence Nationale de la Recherche (French National Research Agency)
LinkOut - more resources
Full Text Sources
Miscellaneous

