Cost-effectiveness of procalcitonin-guided antibiotic duration for hospitalized patients with sepsis
- PMID: 41316366
- PMCID: PMC12661845
- DOI: 10.1186/s13054-025-05732-w
Cost-effectiveness of procalcitonin-guided antibiotic duration for hospitalized patients with sepsis
Abstract
Background: Procalcitonin (PCT)-guided antibiotic duration for critically ill adults with sepsis may be clinically effective and safe. However, cost-effectiveness analyses using clinical trial data for this precision medicine approach in critical care are lacking. This economic evaluation investigates the cost-effectiveness of a daily PCT-guided protocol to guide the duration of antibiotic treatment in adult patients with sepsis.
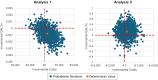
Methods: Two analyses were conducted, the first estimating the cost per quality-adjusted life year (QALY) of the ADAPT-Sepsis study, which recruited 2760 patients randomized to a daily PCT-guided protocol, a daily C-reactive protein-guided protocol and standard care. The second analysis used meta-analyzed results from ADAPT-Sepsis and other PCT-guided treatment studies and employed a lifetime horizon. Key outcomes were the incremental costs and QALYs gained from using the daily PCT-guided protocol approach compared with standard care. Other outcome measures included changes in days of antibiotics, days of hospital stay, days of intensive care unit stay, the percentage of deaths and the number of PCT tests performed.
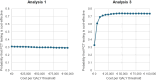
Results: Cost-effectiveness results were driven by the assumed impact of PCT testing on mortality although the confidence/credible intervals for ADAPT-Sepsis and the meta-analyzed data both included no effect. Within ADAPT-Sepsis, the use of PCT tests cost €427 more per patient and was associated with a small QALY loss (0.001), which suggests the daily PCT-guided protocol is dominated. Using meta-analyzed data, the daily PCT-guided protocol was assumed to cost €330 more per patient but was associated with 0.139 more QALYs, resulting in a cost per QALY gained of €2384. If only antibiotic use and PCT tests were assumed to differ then PCT testing is estimated to cost no more than €110 per patient with QALYs equal in both arms regardless of whether ADAPT-Sepsis or meta-analyzed data were used.
Conclusions: This economic analysis has shown that a PCT-guided protocol to guide the duration of antibiotic treatment could be cost-effective. Where only differences in antibiotic use and PCT testing are assumed, the increased costs per patient are modest which may be seen as worthwhile to safely improve antibiotic stewardship for critically ill adult patients with sepsis.
Keywords: Antibiotic duration; Antibiotics; Cost-effectiveness; Health economics; Procalcitonin; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: Dr Dark reported receiving grants from Roche Diagnostics and Abbott via the National Institute of Health and Care Research (NIHR)–agreed contract for biomarker research assay set-up and maintenance in supporting NHS hospitals for which assays are not routinely available, from an NIHR senior investigator award and the NIHR deputy medical director outside the submitted work; and nonfinancial support from Thermo Fisher Scientific via an NIHR memorandum of understanding to assist with knowledge to identify and set up research sites during the conduct of the study.
Figures
References
-
- Wirz Y, Meier M, Bouadma L, Luyt C, Wolff M, Chastre J, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. - DOI - PMC - PubMed
-
- Public health england. start smart—then focus: antimicrobial stewardship toolkit for english hospitals. PHE publications gateway number: 2014828. 2015.
-
- Faculty of intensive care medicine. guidelines for the provision of intensive care services—version 2.1. Faculty of intensive care medicine. 2022.
-
- Dark P, Perkins GD, McMullan R, McAuley D, Gordon AC, Clayton J, et al. BiomArker-guided duration of antibiotic treatment in hospitalised patients with suspecTed sepsis (ADAPT-Sepsis): a protocol for a multicentre randomised controlled trial. J Intensive Care Soc. 2023;24(4):427–34. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials