Low-Dose Colchicine in Patients With Type 2 Diabetes With Recent Myocardial Infarction in the Colchicine Cardiovascular Outcome Trial (COLCOT): A Systematic Review
- PMID: 41322854
- PMCID: PMC12663531
- DOI: 10.7759/cureus.95715
Low-Dose Colchicine in Patients With Type 2 Diabetes With Recent Myocardial Infarction in the Colchicine Cardiovascular Outcome Trial (COLCOT): A Systematic Review
Abstract
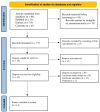
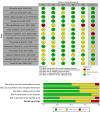
Patients with type 2 diabetes who experience myocardial infarction (MI) have up to a twofold higher risk of recurrent cardiovascular events compared with non-diabetic patients, even under optimal medical therapy. Colchicine, an inexpensive anti-inflammatory agent, has been proposed as an adjunctive therapy for secondary prevention. This systematic review evaluates the efficacy, safety, and cost-effectiveness of low-dose colchicine in this high-risk population, with emphasis on evidence from the COLCOT trial and related randomized controlled studies. A comprehensive search of PubMed, Embase, and the Cochrane Library was performed for studies published between October 2015 and February 2025. Eligible randomized controlled trials (RCTs) evaluated low-dose colchicine (0.5 mg/day) in post-MI patients, including subgroups with type 2 diabetes. Data were extracted on major adverse cardiovascular events (MACE), individual cardiovascular outcomes, adverse effects, and cost-effectiveness. Risk of bias was assessed using the Cochrane RoB 2.0 tool, identifying eight studies with low, four with moderate, and two with high risk of bias. A total of 14 RCTs involving approximately 12,000 participants were included. Colchicine significantly reduced MACE, with hazard ratios ranging from 0.65 to 0.77, and showed consistent benefits in stroke reduction (HR 0.26; 95% CI: 0.10-0.70) and urgent revascularization (HR 0.50; 95% CI: 0.31-0.81). Cost-effectiveness analyses demonstrated a dominant economic profile, lowering healthcare costs while improving quality-adjusted life years (QALYs). The most frequent adverse event was gastrointestinal intolerance, while a modest increase in pneumonia incidence was observed (0.9% vs. 0.4%; P = 0.03). No significant rise in cancer incidence or all-cause mortality was noted. Low-dose colchicine provides substantial cardiovascular protection and cost-effectiveness in post-MI patients, particularly those with type 2 diabetes. Although generally safe and well-tolerated, mild gastrointestinal effects and a slightly elevated infection risk warrant clinical vigilance. Further large-scale, standardized, and long-term trials are essential to confirm its role in contemporary secondary prevention.
Keywords: cardiovascular outcomes; colcot trial; low-dose colchicine; myocardial infarction; type 2 diabetes mellitus.
Copyright © 2025, Shoaib et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Colchicine for the treatment of coronary artery disease. Aimo A, Pascual-Figal DA, Barison A, et al. Trends Cardiovasc Med. 2021;31:497–504. - PubMed
-
- Colchicine in atherosclerotic vascular disease: The good, the bad, and the ugly. Tuvali O, Sella G, Haberman D, Cuciuc V, George J. https://pubmed.ncbi.nlm.nih.gov/35347935/ Isr Med Assoc J. 2022;24:191–197. - PubMed
-
- In patients with type 2 diabetes and recent MI, colchicine reduced a composite CV outcome at 23 mo. Bates ER. Ann Intern Med. 2024;177:0. - PubMed
-
- Colchicine in coronary artery disease: Where do we stand? Bonaventura A, Potere N, Liberale L, Kraler S, Weber BN, Abbate A. J Cardiovasc Pharmacol. 2025;85:243–247. - PubMed
Publication types
LinkOut - more resources
Full Text Sources