Barrier-disrupting microneedle technology: Overcoming physical, physiological, and pathological skin defenses to enhance therapeutic efficacy
- PMID: 41334375
- PMCID: PMC12666373
- DOI: 10.1016/j.bioactmat.2025.11.005
Barrier-disrupting microneedle technology: Overcoming physical, physiological, and pathological skin defenses to enhance therapeutic efficacy
Abstract
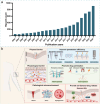
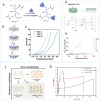
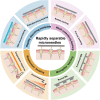
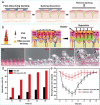
Microneedles (MNs) have gained increasing attention as an advanced transdermal delivery platform owing to their enhanced delivery performance compared to traditional systems. The key to enhancing MNs efficacy lies in overcoming multiple skin barriers that hinder efficient skin insertion and drug permeation: (1) Physical barriers, such as the tough stratum corneum and skin elasticity, impede complete MN insertion; (2) Physiological barriers, including the extracellular matrix (ECM) and biofilms, limit drug diffusion into deeper skin layers for optimal absorption; (3) Pathological microenvironments lead to uncontrollable drug release and insufficient drug accumulation in target tissue. In this review, we outline strategies to address these barriers for improved therapeutic outcomes. First, we discuss optimization approaches for MN geometry, administration methods, and separation performance to enhance skin penetration efficiency. Next, we explore passive (e.g., hyaluronidase, α-amylase) and active (e.g., light, sound, electricity, magnet, and gas) permeation-enhancing strategies to overcome physiological barriers and boost drug bioavailability. Additionally, we examine the design of stimuli-responsive MNs for on-demand drug release in pathological microenvironments, ensuring precise therapeutic effects. Finally, we analyze the challenges and future prospects of MNs-based delivery systems, providing insights to guide their design and clinical application. This review aims to inspire the development of advanced "microneedle warriors" capable of circumventing multiple skin barriers for enhanced therapeutic efficacy.
Keywords: Drug delivery efficiency; Microneedle; Skin barriers; Skin puncture rate; Transdermal permeability.
© 2025 The Authors.
Conflict of interest statement
All authors declare that there is no conflict of interest in this article.
Figures














References
Publication types
LinkOut - more resources
Full Text Sources

