Biomarker-Guided Cardioprotection for Patients Treated With Anthracyclines: A Randomized Clinical Trial
- PMID: 41335439
- PMCID: PMC12676363
- DOI: 10.1001/jamanetworkopen.2025.46201
Biomarker-Guided Cardioprotection for Patients Treated With Anthracyclines: A Randomized Clinical Trial
Abstract
Importance: There are gaps in the understanding of the clinical actionability of cardiovascular biomarkers for risk stratification during cardiotoxic chemotherapy.
Objectives: To gain insights into an N-terminal pro-B-type natriuretic peptide (NT-proBNP)-guided approach for cardioprotection in patients with breast cancer or lymphoma treated with anthracyclines and quantify the feasibility, tolerability, and exploratory efficacy of an NT-proBNP-guided strategy compared with usual care.
Design, setting, and participants: The NT-proBNP guide, a multicenter, randomized (stratified 1:1 by cancer type) clinical trial, included 100 participants with breast cancer or lymphoma initiating anthracyclines from March 18, 2021, to October 20, 2023, who were followed up for 12 months.
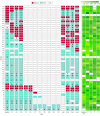
Interventions: Study participants in the NT-proBNP-guided arm had biomarker concentrations measured prior to anthracycline initiation (baseline), at each cycle, and at 3, 6, 9, and 12 months. An elevated NT-proBNP concentration triggered the initiation or titration of neurohormonal therapy. Participants in the usual care arm received routine care without prospective monitoring of NT-proBNP concentrations.
Main outcomes and measures: The primary outcomes were feasibility and safety of the NT-proBNP-guided approach. Feasibility was defined by recruitment, retention, and medication compliance rates. Safety outcomes were assessed according to the Common Terminology Criteria for Adverse Events, version 5.0, at each visit. Exploratory outcomes included differences in blinded, centrally quantified echocardiographic measures and NT-proBNP concentrations between the 2 arms. Analysis was performed on an intention-to-treat approach.
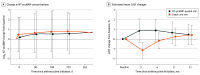
Results: Across 100 participants (mean [SD] age, 52.2 [14.4] years; 86 women [86.0%]), 74 (74.0%) had breast cancer and 26 (26.0%) had lymphoma. At 12 months, the retention rate was 92.7% (89 of 96). In the NT-proBNP-guided arm, 27 participants had NT-proBNP elevations, with a median time from baseline to first elevation of 14 days (IQR, 0-76 days), and a median time between the first NT-proBNP elevation and neurohormonal therapy prescription of 1 day (IQR, 0.5-9 days). There were no significant differences in targeted adverse events between the NT-proBNP-guided (23 events) and usual care (16 events) arms (P = .13). At 3 months, left ventricular ejection fraction (LVEF) was modestly higher in the NT-proBNP-guided arm compared with usual care (mean difference, 2.0% [95% CI, 0.5%-3.5%]; P = .007). NT-proBNP concentrations increased in both arms over the study duration, but elevations were slightly attenuated in the NT-proBNP-guided arm.
Conclusions and relevance: This randomized clinical trial of an NT-proBNP-guided approach to neurohormonal therapy in patients with cancer treated with anthracyclines demonstrates the feasibility, safety, and potential modest, early improvement in LVEF of a biomarker-guided approach. These findings provide support for further study of an NT-proBNP-guided approach to cardioprotection for patients undergoing cancer treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT04737265.
Conflict of interest statement
Figures

References
-
- Lewinter C, Nielsen TH, Edfors LR, et al. A systematic review and meta-analysis of beta-blockers and renin-angiotensin system inhibitors for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer. Eur Heart J. 2022;43(27):2562-2569. doi: 10.1093/eurheartj/ehab843 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

