Longitudinal Blood-Based Biomarkers and Clinical Progression in Subjective Cognitive Decline
- PMID: 41335440
- PMCID: PMC12676364
- DOI: 10.1001/jamanetworkopen.2025.45862
Longitudinal Blood-Based Biomarkers and Clinical Progression in Subjective Cognitive Decline
Abstract
Importance: Blood-based biomarkers identify Alzheimer disease and hold promise for monitoring disease progression, even in the preclinical disease stages.
Objective: To investigate longitudinal trajectories of blood-based biomarkers and association with cognitive decline and risk of progression in individuals with subjective cognitive decline (SCD).
Design, setting, and participants: This prospective cohort study (Subjective Cognitive Impairment Cohort) of individuals with SCD evaluated at a memory clinic underwent biennial biomarker collection and annual cognitive assessment and diagnostic evaluation from January 1, 2005, to December 31, 2023, with follow-up through 2023.
Exposure: Amyloid status was determined using positron emission tomography or cerebrospinal fluid. Plasma Aβ42/40, phosphorylated tau 217 (pTau217), glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) were measured biennially.
Main outcomes and measures: Cognitive trajectories in memory, attention, language, executive function, and global cognition and clinical progression to mild cognitive impairment or dementia.
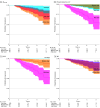
Results: A total of 298 individuals (mean [SD] age, 61.55 [8.08] years; 174 [58.4%] male) with SCD were included, of whom 80 were amyloid-positive (A+) and 218 were amyloid-negative (A-). Mean (SD) follow-up was 4.8 (2.6) years. Individuals with SCD A+ were older (mean [SD] age, 65.25 [7.14] years; 42 [52.5%] male) than those with SCD A- (mean [SD] age, 60.19 [8.00] years; 132 [60.6%] male). For pTau217, GFAP, and NfL, baseline levels were higher in the A+ group compared with A- group (estimates [SE] amyloid β, 1.11 [0.11], 0.69 [0.13], and 0.36 [0.10], respectively; P < .001 for all). Additionally, these biomarkers showed steeper increases over time in the A+ group than in A- group (estimates [SE] time × amyloid status β, 0.07 [0.02], P < .001; 0.07 [0.02], P < .001; and 0.05 [0.02], P = .005, respectively). Longitudinal increases in pTau217 and GFAP were associated with cognitive decline over time in all domains (β time × biomarker slope = -0.02 to -0.04). Longitudinal decreases in Aβ42/40 and increases in NfL were associated with cognitive decline in global cognition (β = 0.03 [0.01], P = .04) and language (β = 0.04 [0.02], P = .03), and increases in NfL were also associated with decline in global cognition (β = -0.02 [0.01], P = .004), language (β = -0.03 [0.01], P = .007), and executive functioning (β = -0.03 [0.01], P = .02). Steeper pTau217 slope was associated with progression from SCD to mild cognitive impairment or dementia (hazard ratio [HR], 3.6; 95% CI, 1.8-7.4 per 0.05 SD increase per year; C index, 0.89; 95% CI, 0.84-0.93), as were steeper GFAP slope (HR, 1.5 [95% CI, 1.0-2.2]; C index, 0.81 [95% CI, 0.73-0.88]) and steeper NfL slope (HR, 2.6 [95% CI, 1.3-5.2]; C index, 0.77 [95% CI, 0.69-0.85]). Aβ42/40 slope was not associated with progression.
Conclusions and relevance: This cohort study of individuals with SCD suggests that longitudinal plasma pTau217 and GFAP are suitable biomarkers for monitoring AD pathology. Their changes were associated with cognitive decline and clinical progression, supporting their potential utility for early intervention and disease monitoring.
Conflict of interest statement
Figures


References
-
- Shao K, Hu X, Buerger K, et al. Longitudinal cognitive changes in SCD participants in relation to plasma amyloid status and the potential additive effect of plasma ptau181 levels: a CLoCODE study. Alzheimers Dement. 2025;20(uppl 2):e086729. doi: 10.1002/alz.086729 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

