Above the Energy Gap Law: Heavy Chalcogenide Substitution in NIR II-Emissive Diradicaloid Qubits
- PMID: 41341053
- PMCID: PMC12670300
- DOI: 10.1021/acscentsci.5c01001
Above the Energy Gap Law: Heavy Chalcogenide Substitution in NIR II-Emissive Diradicaloid Qubits
Abstract
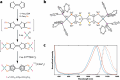
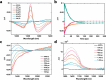
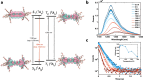
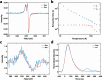
Near-infrared (NIR, 700-1700 nm)- and telecom (∼1260-1625 nm)-emissive molecules are good candidates for biological imaging and quantum sensing applications, respectively; however, bright low energy emission is rare due to exponentially increasing nonradiative decay rates in these regions, a phenomenon known as the energy gap law. Recent literature has emphasized the importance of minimizing skeletal modes to prevent increased nonradiative decay rates, but most organic lumiphores in these regions utilize large, conjugated scaffolds containing many CC modes. Here we report a compact, telecom-emissive scaffold, tetrathiafulvalene-2,3,6,7-tetraselenate, or TTFts, that displays remarkable air, water, and acid stability, exhibits record quantum yields and brightness values, and retains quantum coherence under ambient conditions. These properties are enabled through methodical selenium substitution, which bathochromically shifts emission while shifting skeletal vibrations to lower energies. This new scaffold validates heavy heteroatom substitution strategies and establishes a new class of bright telecom emitters and robust qubits.
© 2025 The Authors. Published by American Chemical Society.
Figures

References
-
- Hong G., Antaris A. L., Dai H.. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed Eng. 2017;1(1):0010. doi: 10.1038/s41551-016-0010. - DOI
-
- Matsui A., Tanaka E., Choi H. S., Winer J. H., Kianzad V., Gioux S., Laurence R. G., Frangioni J. V.. Real-Time Intra-Operative near-Infrared Fluorescence Identification of the Extrahepatic Bile Ducts Using Clinically Available Contrast Agents. Surgery. 2010;148(1):87–95. doi: 10.1016/j.surg.2009.12.004. - DOI - PMC - PubMed
-
- Li B., Zhao M., Feng L., Dou C., Ding S., Zhou G., Lu L., Zhang H., Chen F., Li X., Li G., Zhao S., Jiang C., Wang Y., Zhao D., Cheng Y., Zhang F.. Organic NIR-II Molecule with Long Blood Half-Life for in Vivo Dynamic Vascular Imaging. Nat. Commun. 2020;11(1):3102. doi: 10.1038/s41467-020-16924-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
